- Record: found
- Abstract: found
- Article: found
TEAD1 (TEA Domain Transcription Factor 1) Promotes Smooth Muscle Cell Proliferation Through Upregulating SLC1A5 (Solute Carrier Family 1 Member 5)-Mediated Glutamine Uptake
Read this article at
Abstract
Supplemental Digital Content is available in the text.
Abstract
Rationale:
TEAD (TEA domain transcription factor) 1—a major effector of the Hippo signaling pathway—acts as an oncoprotein in a variety of tumors. However, the function of TEAD1 in vascular smooth muscle cells (VSMCs) remains unclear.
Objective:
To assess the role of TEAD1 in vascular injury–induced smooth muscle proliferation and delineate the mechanisms underlying its action.
Methods and Results:
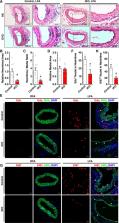
We found that TEAD1 expression is enhanced in mouse femoral artery after wire injury and correlates with the activation of mTORC1 (mechanistic target of rapamycin complex 1) signaling in vivo. Using an inducible smooth muscle–specific Tead1 KO (knockout) mouse model, we found that specific deletion of Tead1 in adult VSMCs is sufficient to attenuate arterial injury–induced neointima formation due to inhibition of mTORC1 activation and VSMC proliferation. Furthermore, we found that TEAD1 plays a unique role in VSMCs, where it not only downregulates VSMC differentiation markers but also activates mTORC1 signaling, leading to enhanced VSMC proliferation. Using whole-transcriptome sequencing analysis, we identified Slc1a5 (solute carrier family 1 member 5)—a key glutamine transporter—as a novel TEAD1 target gene. SLC1A5 overexpression mimicked TEAD1 in promoting mTORC1 activation and VSMC proliferation. Moreover, depletion of SLC1A5 by silencing RNA or blocking SLC1A5-mediated glutamine uptake attenuated TEAD1-dependent mTORC1 activation and VSMC proliferation.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Bidirectional transport of amino acids regulates mTOR and autophagy.
- Record: found
- Abstract: found
- Article: not found
Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery.
- Record: found
- Abstract: found
- Article: not found