- Record: found
- Abstract: found
- Article: found
Real-World Experience of People with Hemophilia A Receiving Turoctocog Alfa Pegol (N8-GP): Results from a Patient Experience Survey
Read this article at
Abstract
Purpose
Turoctocog alfa pegol (N8-GP) is an extended half-life recombinant factor VIII molecule used for the treatment of hemophilia A (HA). The purpose of this study was to investigate real-world experiences of patients with HA treated with N8-GP.
Patients and Methods
A 25-minute online survey was completed by adults (≥18 years) and caregivers of adolescents (12–16 years) with HA receiving N8-GP across six countries (Germany, Italy, Portugal, Spain, UK and US). Patients were recruited using a multichannel approach through recruitment panels, referrals from healthcare professionals and patient associations. The survey comprised a questionnaire with metrics including satisfaction and preferences for N8-GP, quality of life (QoL) and long-term impact.
Results
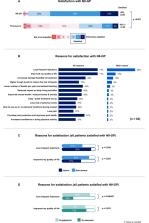
A total of 62 participants (98% male [n=61], mean age 29 years) comprising 46 patients and 16 caregivers completed the survey. Patients (60% non-severe [n=37] and 40% severe [25]) were on N8-GP for a mean period of 1.4 years. Patients expressed satisfaction (95% vs 42%, p<0.001) and preference (91% vs 9%, p<0.001) for N8-GP vs their previous treatments. Most patients with severe HA (87%, p=0.038) and patients on prophylaxis (84%, p<0.001) stated lower frequency of injections as their main reason for satisfaction, while improved QoL drove satisfaction for non-severe patients (81%, p=0.053). Overall, patients perceived that QoL score improved (74.8 vs 65.9, p=0.01) with N8-GP treatment compared with previous treatments. Flexibility to store at room temperature was one of the key convenience factors driving satisfaction. Patients believed that N8-GP can offer a long-term impact in areas such as ability to perform day-to-day activities (68%), independence to live like a person without hemophilia (63%), ability to travel (60%) with a feeling of optimism and hopefulness (82%).
Plain language summary
Hemophilia A is a rare bleeding disorder that often runs in families. Although there is no cure, several therapeutic options are available to help control bleeding in people with hemophilia A. However, most treatments require intravenous (directly into a vein) or subcutaneous (directly under the skin) injections, which is a significant burden for all patients. In this study, adults and caregivers of adolescents, with hemophilia A answered a survey about their treatment experience with a medicine called N8-GP (turoctocog alfa pegol) compared with previous treatments.
This is the first real-world evidence study focused on satisfaction with and preference for N8-GP compared with previous treatments. Survey results showed that adults and adolescents with hemophilia A were very satisfied with N8-GP compared with previous medicines. The main reasons for satisfaction included less frequent injections, the flexibility to store at room temperature, and improved quality-of-life.
In addition, many patients expressed hopes for the future while taking N8-GP, such as confidence in ability to undertake physical activities and ability to plan and go on a holiday. Compared with previous treatments, patients were feeling optimistic and hopeful about N8-GP and expressed that they have started to think less about the disease.
Despite the unique advantages of taking N8-GP, patients overall still perceive their quality-of-life to be less than an average person’s, presenting an opportunity for future advancements. Overall, this study sheds light on the unique experiences of people with hemophilia A taking N8-GP and further opens up the scope for addressing the unmet needs.
Related collections
Most cited references19
- Record: found
- Abstract: not found
- Article: not found
WFH Guidelines for the Management of Hemophilia, 3rd edition
- Record: found
- Abstract: found
- Article: found
Trial designs using real‐world data: The changing landscape of the regulatory approval process

- Record: found
- Abstract: found
- Article: found