- Record: found
- Abstract: found
- Article: found
Spesolimab, an Anti-Interleukin-36 Receptor Antibody, in Patients with Palmoplantar Pustulosis: Results of a Phase IIa, Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Study
Read this article at
Abstract
Introduction
Palmoplantar pustulosis (PPP) is a chronic, inflammatory skin disease, with high disease burden, that is often refractory to treatment. There is a high unmet clinical need for the treatment of patients with PPP. The objectives of this study were to evaluate the safety and efficacy of spesolimab, a novel anti-interleukin-36 receptor antibody, in patients with PPP.
Methods
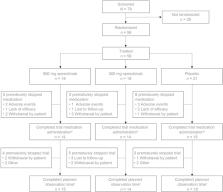
This was a phase IIa, multicenter, double-blind, randomized, placebo-controlled pilot study comparing 900 mg spesolimab ( n = 19), 300 mg spesolimab ( n = 19), and placebo ( n = 21) administered intravenously every 4 weeks until week 12 in patients with PPP. The primary efficacy endpoint was the achievement of Palmoplantar Pustulosis Area and Severity Index 50 (PPP ASI50) at week 16, defined as achieving an ≥ 50% decrease from baseline PPP ASI.
Results
At week 16, 31.6% of patients in both spesolimab dose groups achieved PPP ASI50 versus 23.8% receiving placebo (risk difference 0.078; 95% confidence interval –0.190, 0.338). Thus, the primary endpoint was not met. Spesolimab was well tolerated with no clinically relevant treatment-emergent safety signals observed.
Conclusions
PPP severity declined over time in all treatment groups after the start of treatment, with a faster decline in the spesolimab arms than in the placebo arm, indicating a potential treatment effect for spesolimab. Limitations to the study included a small sample size and lower overall disease severity than expected at baseline. It is possible that the primary efficacy endpoint may have coincided with natural disease resolution in some patients. Further effects of the efficacy of spesolimab in PPP are being explored in a phase IIb trial.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis.
- Record: found
- Abstract: found
- Article: not found
Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis.
- Record: found
- Abstract: found
- Article: not found