- Record: found
- Abstract: found
- Article: found
Anesthetic Isoflurane Increases Phosphorylated Tau Levels Mediated by Caspase Activation and Aβ Generation
Read this article at
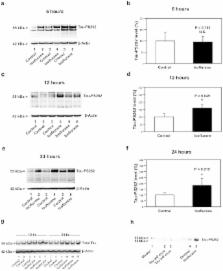
Abstract
Anesthetic isoflurane has been shown to promote Alzheimer’s disease (AD) neuropathogenesis by inducing caspase activation and accumulation of β-amyloid (Aβ). Phosphorylation of tau protein is another important feature of AD neuropathogenesis. However, the effects of isoflurane on phosphorylated tau levels remain largely to be determined. We therefore set out to determine whether isoflurane can increase phosphorylated tau levels. 5 to 8 month-old wild-type and AD transgenic mice [B6.Cg-Tg (APPswe, PSEN1dE9)85Dbo/J] were treated with 1.4% isoflurane for two hours. The mice brain tissues were harvested at six, 12 and 24 hours after the anesthesia. For the in vitro studies, primary neurons from wild-type and the AD transgenic mice were exposed to 2% isoflurane for six hours, and were harvested at the end of anesthesia. The harvested brain tissues and neurons were subjected to Western blot analysis by which the levels of phosphorylated tau protein at Serine 262 (Tau-PS262) were determined. Here we show that the isoflurane anesthesia increased Tau-PS262 levels in brain tissues and primary neurons from the wild-type and AD transgenic mice. Moreover, the isoflurane anesthesia may induce a greater increase in Tau-PS262 levels in primary neurons and brain tissues from the AD transgenic mice. Finally, caspase activation inhibitor Z-VAD and Aβ generation inhibitor L-685,458 attenuated the isoflurane-induced increases in Tau-PS262 levels. In conclusion, clinically relevant isoflurane anesthesia increases phosphorylated tau levels, which may result from the isoflurane-induced caspase activation and Aβ generation. These findings will promote more studies to determine the effects of anesthetics on tau phosphorylation.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Tau protein isoforms, phosphorylation and role in neurodegenerative disorders.
- Record: found
- Abstract: found
- Article: not found
Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis.
- Record: found
- Abstract: found
- Article: not found