- Record: found
- Abstract: found
- Article: found
Neutrophils enhance early Trypanosoma brucei infection onset
Read this article at
Abstract
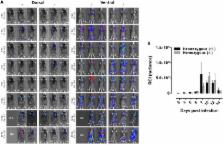
In this study, Trypanosoma brucei was naturally transmitted to mice through the bites of infected Glossina morsitans tsetse flies. Neutrophils were recruited rapidly to the bite site, whereas monocytes were attracted more gradually. Expression of inflammatory cytokines ( il1b, il6), il10 and neutrophil chemokines ( cxcl1, cxcl5) was transiently up-regulated at the site of parasite inoculation. Then, a second influx of neutrophils occurred that coincided with the previously described parasite retention and expansion in the ear dermis. Congenital and experimental neutropenia models, combined with bioluminescent imaging, indicate that neutrophils do not significantly contribute to dermal parasite control and elicit higher systemic parasitemia levels during the infection onset. Engulfment of parasites by neutrophils in the skin was rarely observed and was restricted to parasites with reduced motility/viability, whereas live parasites escaped phagocytosis. To our knowledge, this study represents the first description of a trypanosome infection promoting role of early innate immunological reactions following an infective tsetse fly bite. Our data indicate that the trypanosome is not hindered in its early development and benefits from the host innate responses with the neutrophils being important regulators of the early infection, as already demonstrated for the sand fly transmitted Leishmania parasite.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Leishmaniasis: complexity at the host-pathogen interface.

- Record: found
- Abstract: found
- Article: found
Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice
- Record: found
- Abstract: found
- Article: not found