- Record: found
- Abstract: found
- Article: found
Pauson-Khand Reaction of Internal Dissymmetric Trifluoromethyl Alkynes. Influence of the Alkene on the Regioselectivity
research-article
Nuria Aiguabella
1 ,
Elsa M. Arce
1 ,
Carlos del Pozo
2
,
3 ,
Xavier Verdaguer
1
,
4 ,
Antoni Riera
1
,
4
,
*
03 February 2014
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
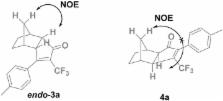
The scope of the Pauson-Khand reaction (PKR) of internal trifluoromethyl alkynes, previously described with norbornadiene, is expanded to norbornene and ethylene. A thorough structural analysis of the resulting PK adducts has been carried out to unveil that α-trifluoromethylcyclopentenones are preferred in all cases, independently of the electronic properties of the alkyne. The regioselectivity observed with norbornadiene and ethylene is higher than in the case of norbornene.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Introduction of fluorine and fluorine-containing functional groups.
- Record: found
- Abstract: not found
- Article: not found
Direct Trifluoromethylation of the CH Bond
Hui Liu, Zhenhua Gu, Xuefeng Jiang (2013)
- Record: found
- Abstract: found
- Article: not found
Copper-mediated aerobic oxidative trifluoromethylation of terminal alkynes with Me3SiCF3.
Feng-Ling Qing, Lingling Chu (2010)