- Record: found
- Abstract: found
- Article: not found
COVID-19 transmission following outpatient endoscopy during pandemic acceleration phase involving SARS-CoV-2 VOC 202012/01 variant in UK
research-article
Bu'Hussain Hayee
1
,
,
The SCOTS II Project group,
Pradeep Bhandari
2 ,
Colin J Rees
3 ,
Ian Penman
4
(Collab),
Vivienne Sayer (Collab),
Mayur Kumar (Collab),
Kath Lynch (Collab),
Olaolu Olabintan Ben Warner (Collab),
Imogen Sutherland (Collab),
Gabor Sipos (Collab),
Zacharias Tsiamoulos (Collab),
Shraddha Gulati (Collab),
Mehul Patel (Collab),
Ed Seward (Collab),
Rawen Kader (Collab),
Sergio Coda (Collab),
Sas Banerjee (Collab),
Adam Humphries (Collab),
Sarah Marshall (Collab),
Angad Dhillon (Collab),
Romanov Nable (Collab)
24 March 2021
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Message
Infection prevention and control (IPC) measures put in place during the first phases
of the COVID-19 pandemic were effective in reducing endoscopy-related transmission
while allowing recovery of activity.
In late 2020 a novel, more infectious, SARS-CoV-2 variant (VOC 202012/01) was associated
with a second ’surge' or acceleration phase in the UK. We sought to measure whether
pre-existing IPC guidance would be sufficient to prevent transmission in this scenario.
Prospective data were collected from eight UK centres for n=2440 procedures. Pre-endoscopy,
nine (0.37%) asymptomatic patients were positive for SARS-CoV-2 by nasopharyngeal
swab (NPS) testing and their procedures deferred. Post endoscopy, 30 (1.27%) developed
symptoms suspicious for COVID-19, with 15 (0.65%) testing positive on NPS. Three (0.12%)
cases were attributed to potential transmission from endoscopy attendance. All 15
patients recovered fully requiring only community treatment.
Although we report cases potentially transmitted by endoscopy attendance in this latest
study, the risk of COVID-19 transmission following outpatient endoscopy remains very
low. Thus, IPC measures developed in earlier pandemic phases appear robust, but our
data emphasise the need for vigilance and strict adherence to these measures in order
to optimally protect both patients and staff.
In more detail
The effects of the COVID-19 pandemic continue to extend beyond direct care of affected
patients,1 particularly impacting outpatient diagnostics including GI endoscopy. Considerable
concerns remain around the potential impact on detection of, and survival from, significant
disease such as cancer.2 3 In mid-2020, a pandemic deceleration phase4 in the UK led
to a period of intense ‘restart and recovery’ activity in endoscopy to mitigate the
effects of delayed or cancelled procedures. This was supported by professional society
guidance on the development of ‘COVID-minimised’ or ‘green’ pathways with NPS testing
of patients before their attendance for the procedure.5–7 Activity was limited by
the impact on endoscopy staff and resources, but additionally by patient concerns
regarding the risk of transmission by attending hospital; a complex and multifactorial
challenge.8 9 A multicentre study of COVID-19 transmission following outpatient endoscopy
in the deceleration phase (when community infection rates were low) demonstrated that,
with appropriate IPC measures in place,5 10 there were no recorded cases of transmission
in over 6200 patients.11
In early December 2020, the effect of a new SARS-Cov-2 variant (termed VOC 202012/01)
was associated with an acceleration phase in southeast England.12 13 Pre-existing
IPC measures had been developed to facilitate safe endoscopy during a pandemic deceleration
or recovery phase (with relatively low rates of community infection).7 These comprised
telephone screening for COVID-19 symptoms; pre-procedure NPS testing (even in asymptomatic
individuals); separation of pathways according to perceived or actual transmission
risk and the potential for aerosol generation. Furthermore, a variety of testing strategies,
with varying levels of accuracy, have been employed across hospitals in the UK and
internationally.14–16 Despite these concerns, the negative predictive value (NPV),
even of an imperfect test, was felt to be sufficiently high to rely on NPS as a cornerstone
of the ‘green’ pathway.7 As NPV is dependent on prevalence, we sought to determine
whether IPC measures were sufficient to prevent COVID-19 transmission during an acceleration
pandemic phase, with rising prevalence as well as a more infectious viral variant.
This multicentre prospective study collected data from consecutive outpatients attending
for elective diagnostic or therapeutic endoscopy from eight centres across southeast
England. No patient identifiable data were collected, no treatment decisions were
affected and no identifiable data were analysed or transferred. Review by the Research
Governance committee at the lead author’s institution confirmed that ethical approval
was not required. Participating centres were invited to submit data for the 3-week
period 14–31 December 2020 inclusive, based on the identification of an acceleration
phase as above, with rising community incidence in the areas served by those hospitals
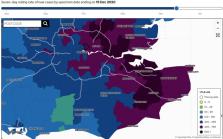
(at least 800 cases per 100 000 population per week; figure 1; compared with <10 per
100 000 in August 202013 17). These were three London tertiary care hospitals, two
London secondary care hospitals and three secondary care hospitals in southeast England
(the county of Kent adjacent to London).
Figure 1
Rolling-rate of new cases in the regions served by the hospitals participating in
this study (as of 15 December 2020). Downloaded with permission from Ref. 13. Rates
are per 100 000 population.
All centres prospectively completed an anonymised database of patients including procedure
type, responses to preprocedure criteria,7 preprocedural NPS result, source of referral
and dates for all activities. All centres conducted patient follow-up by telephone
consultation at 7 and 14 days after the procedure to check for symptoms of COVID-19.
If symptoms were reported, all patients who had not already been tested based on their
development of symptoms underwent NPS testing according to local or national protocols
and the results were recorded. In all cases, regardless of NPS result, the outcome
of symptoms was noted and, in cases testing positive for SARS-CoV-2, a root-cause
analysis was performed by the reporting hospital to determine the most likely source
of transmission. The mean incubation period for COVID-19 is understood to be around
5 days.18–20 In order to be attributed to transmission in the endoscopy unit, therefore,
patients must have developed symptoms within 10 days of attendance and have no other
more likely source of transmission identified on direct questioning by the local care
team.
Data were collected from n=2440 (48.8% female) patients undergoing diagnostic or therapeutic
endoscopy (n=966 (39.6%) upper endoscopy; figure 2).
Figure 2
Proportions of procedures performed.
Before endoscopy, 9/2449 (0.37%) asymptomatic patients were positive for SARS-CoV-2
and had their procedures deferred. These nine patients were not included in further
analysis. After endoscopy, 30/2440 (1.27%) developed symptoms suspicious for COVID-19,
with 16 (0.65%) testing positive on NPS. All cases recovered without the need for
hospital admission. After analysis, there were three (0.12%) cases where no other
likely source of transmission was identified, other than the attendance for endoscopy
(table 1). There were no cases of transmission to staff members as a direct result
of these cases. It was not possible to calculate overall rates of infection in staff
as the number of staff in units was highly variable with significant rotation due
to secondment or redeployment, but there were only six confirmed cases in staff members
across all participating sites. Rates of staff absence varied considerably, with three
hospitals (two tertiary and one secondary care) reporting no absence due to COVID-19
in the 3-week period of the study. One hospital reported absence of nearly 75% of
its endoscopy staff due to two infected staff members (from community transmission),
mandating isolation for the others while testing was performed. This was primarily
due to uncertainty around adherence to IPC measures in a break room. No COVID-19 cases
in either patients or staff required hospitalisation or additional treatment and all
resolved without further event.
Table 1
Analysis of COVID-19 cases confirmed by nasopharyngeal swab (NPS) after symptom onset
Case
Hospital
Total endoscopy activity (cases)
Procedure
Days from endoscopy to symptom onset
Cause identified on review
Attributed to endoscopy?
1
A
440
Colonoscopy
12
Attended for CT scan on day 5 after endoscopy (non-swab)
No
2
B
458
OGD
7
No other likely source identified
Yes
3
Colonoscopy
5
Attended emergency department on day prior to endoscopy
No
4
C
263
Colonoscopy
6
No other likely source identified
Yes
5
Colonoscopy
3
Family member with confirmed infection prior to attendance*
No
6
Sigmoidoscopy
2
Multiple family members with confirmed infection†
No
7
Colonoscopy
4
Attended for CT scan 3 days prior to endoscopy (non-swab)
No
8
D
462
ERCP
5
Temporary admission to ward where outbreak occurred
No
9
Colonoscopy
2
Family member had confirmed infection prior to attendance*
No
10
OGD
5
Family member had confirmed infection prior to attendance*
No
11
Colonoscopy
8
Hospital staff; returned to work immediately after endoscopy
No
12
Colonoscopy
2
Family member had confirmed infection prior to attendance*
No
13
E
194
ERCP
13
Family member had confirmed infection after attendance*
No
14
ERCP
11
No other likely source identified
Yes
15
OGD
1
Family member had confirmed infection prior to attendance*
No
16
F
472
OGD
4
NHS employee (administrative) with multiple duties in hospital
No
2 – secondary care; 3 – tertiary care; *; .
*Cases known only in retrospect, between preprocedure NPS and attendance.
†History not disclosed by patient prior to attendance (preprocedure telephone questionnaire).
ERCP, endoscopic retrograde pancreatography; OGD, oesophagogastroduodenoscopy.
Comments
This multicentre prospective study of 2440 patients undertaken during a pandemic acceleration
phase of a more infectious SARS-Cov-2 variant provides reassurance that GI endoscopy
is associated with a very low risk of transmission for both patients and staff.
While asymptomatic positive rates are higher than the previous study,11 the rate remains
low, at less than 0.5%. The risk of acquiring COVID-19 from endoscopy continues to
remain very low. However, it is important to acknowledge that this rate is not zero.
This serves to emphasise the need for vigilance and strict adherence to the principle
of a COVID-minimised pathway.
The risk of missed or delayed cancer diagnosis would appear to significantly outweigh
the risks of COVID-19 transmission. We believe these data should be of continued reassurance
to healthcare providers and patients alike, facilitating the provision of much-needed
endoscopy services.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application
Stephen A. Lauer, Kyra Grantz, Qifang Bi … (2020)

- Record: found
- Abstract: found
- Article: found
The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study
Camille Maringe, James Spicer, Melanie Morris … (2020)

- Record: found
- Abstract: found
- Article: found
Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020
Jantien Backer, Don Klinkenberg, Jacco Wallinga (2020)
