- Record: found
- Abstract: found
- Article: found
Exploring Biogeochemistry and Microbial Diversity of Extant Microbialites in Mexico and Cuba
Read this article at
Abstract
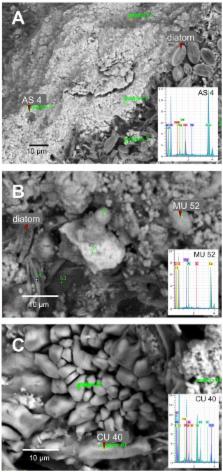
Microbialites are modern analogs of ancient microbial consortia that date as far back as the Archaean Eon. Microbialites have contributed to the geochemical history of our planet through their diverse metabolic capacities that mediate mineral precipitation. These mineral-forming microbial assemblages accumulate major ions, trace elements and biomass from their ambient aquatic environments; their role in the resulting chemical structure of these lithifications needs clarification. We studied the biogeochemistry and microbial structure of microbialites collected from diverse locations in Mexico and in a previously undescribed microbialite in Cuba. We examined their structure, chemistry and mineralogy at different scales using an array of nested methods including 16S rRNA gene high-throughput sequencing, elemental analysis, X-Ray fluorescence (XRF), X-Ray diffraction (XRD), Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM-EDS), Fourier Transformed Infrared (FTIR) spectroscopy and Synchrotron Radiation-based Fourier Transformed Infrared (SR-FTIR) spectromicroscopy. The resulting data revealed high biological and chemical diversity among microbialites and specific microbe to chemical correlations. Regardless of the sampling site, Proteobacteria had the most significant correlations with biogeochemical parameters such as organic carbon (C org), nitrogen and C org:Ca ratio. Biogeochemically relevant bacterial groups (dominant phototrophs and heterotrophs) showed significant correlations with major ion composition, mineral type and transition element content, such as cadmium, cobalt, chromium, copper and nickel. Microbial-chemical relationships were discussed in reference to microbialite formation, microbial metabolic capacities and the role of transition elements as enzyme cofactors. This paper provides an analytical baseline to drive our understanding of the links between microbial diversity with the chemistry of their lithified precipitations.
Related collections
Most cited references126
- Record: found
- Abstract: found
- Article: not found
FLASH: fast length adjustment of short reads to improve genome assemblies.
- Record: found
- Abstract: found
- Article: not found
CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution.
- Record: found
- Abstract: not found
- Article: not found