- Record: found
- Abstract: found
- Article: not found
Effects of Kras activation and Pten deletion alone or in combination on MUC1 biology and epithelial to mesenchymal transition in ovarian cancer
Read this article at
Abstract
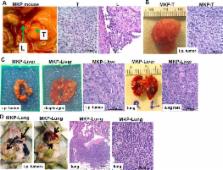
Mucin1 (MUC1) is an epithelial glycoprotein overexpressed in ovarian cancer and actively involved in tumor cell migration and metastasis. Using novel in vitro and in vivo MUC1-expressing conditional (Cre-loxP) ovarian tumor models, we focus here on MUC1 biology and the roles of Kras activation and Pten deletion during cell transformation and epithelial-to-mesenchymal transition (EMT). We generated several novel murine ovarian cancer cell lines derived from the ovarian surface epithelia (OSE) of mice with conditional mutations in Kras, Pten or both. In addition, we also generated several tumor-derived new cell lines that reproduce the original tumor phenotype in vivo and mirror late stage metastatic disease.
Our results demonstrate that de novo activation of oncogenic Kras does not trigger increased proliferation, cellular transformation or EMT and prevents MUC1 upregulation. In contrast, Pten deletion accelerates cell proliferation, triggers cellular transformation in vitro and in vivo and stimulates MUC1 expression. Ovarian tumor-derived cell lines MKP-Liver and MKP-Lung cells reproduce in vivo EMT and represent the first immune competent mouse model for distant hematogenous spread. Whole genome microarray expression analysis using tumor and OSE-derived cell lines reveals a 121 gene signature associated with EMT and metastasis. When applied to n=542 cases from the ovarian cancer TCGA dataset, the gene signature identifies a patient subset with decreased survival (p=0.04). Using an extensive collection of novel murine cell lines we have identified distinct roles for Kras and Pten on MUC1 and EMT in vivo and in vitro. The data has implications for future design of combination therapies targeting Kras mutations, Pten deletions and MUC1 vaccines.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: found
Ovarian cancer
- Record: found
- Abstract: found
- Article: not found
