- Record: found
- Abstract: found
- Article: found
Selective Deletion of Heparan Sulfotransferase Enzyme, Ndst1, in Donor Endothelial and Myeloid Precursor Cells Significantly Decreases Acute Allograft Rejection
Read this article at
Abstract
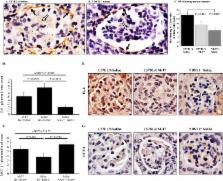
Early damage to transplanted organs initiates excess inflammation that can cause ongoing injury, a leading cause for late graft loss. The endothelial glycocalyx modulates immune reactions and chemokine-mediated haptotaxis, potentially driving graft loss. In prior work, conditional deficiency of the glycocalyx-modifying enzyme N-deacetylase-N-sulfotransferase-1 (Ndst1 f/f TekCre +) reduced aortic allograft inflammation. Here we investigated modification of heparan sulfate (HS) and chemokine interactions in whole-organ renal allografts. Conditional donor allograft Ndst1 deficiency ( Ndst1 −/−; C57Bl/6 background) was compared to systemic treatment with M-T7, a broad-spectrum chemokine-glycosaminoglycan (GAG) inhibitor. Early rejection was significantly reduced in Ndst1 −/− kidneys engrafted into wildtype BALB/c mice ( Ndst1 +/+) and comparable to M-T7 treatment in C57Bl/6 allografts (P < 0.0081). M-T7 lost activity in Ndst1 −/− allografts , while M-T7 point mutants with modified GAG-chemokine binding displayed a range of anti-rejection activity. CD3+ T cells (P < 0.0001), HS (P < 0.005) and CXC chemokine staining (P < 0.012), gene expression in NFκB and JAK/STAT pathways, and HS and CS disaccharide content were significantly altered with reduced rejection. Transplant of donor allografts with conditional Ndst1 deficiency exhibit significantly reduced acute rejection, comparable to systemic chemokine-GAG inhibition. Modified disaccharides in engrafted organs correlate with reduced rejection. Altered disaccharides in engrafted organs provide markers for rejection with potential to guide new therapeutic approaches in allograft rejection.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
The endothelial glycocalyx: composition, functions, and visualization
- Record: found
- Abstract: found
- Article: not found
Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines.
- Record: found
- Abstract: found
- Article: not found