- Record: found
- Abstract: found
- Article: found
IL-17: an important pathogenic factor in endometriosis
Read this article at
Abstract
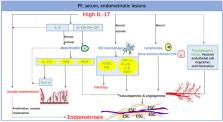
Interleukin-17 (IL-17) is known as a Th17-cell-derived proinflammatory cytokine, which plays a pivotal role in several inflammatory and autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and psoriasis. Emerging evidence has shown that IL-17 is linked to endometriosis, although the etiology of endometriosis is still unknown. The IL-17 expression is up-regulated in serum, peritoneal fluid (PF) and endometriotic lesions from patients with endometriosis but the related regulation mechanisms are complex and obscure. Meanwhile, the specific roles of IL-17 in endometriosis are also worthy of further exploration. Through the integration and summary of literature, we conclude that the secretion of IL-17 increases under the regulation of ectopic microenvironment and other factors, and then IL-17 is deeply involved in endometriosis in the regulation of immune microenvironment, the invasion and growth of ectopic lesions, and so on, which implies its therapeutic value in this disorder.
Related collections
Most cited references145
- Record: found
- Abstract: found
- Article: not found
A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17.
- Record: found
- Abstract: found
- Article: not found
Macrophages in Tissue Repair, Regeneration, and Fibrosis.
- Record: found
- Abstract: found
- Article: not found