- Record: found
- Abstract: found
- Article: not found
Drug-induced caspase 8 upregulation sensitises cisplatin-resistant ovarian carcinoma cells to rhTRAIL-induced apoptosis
Read this article at
Abstract
Background:
Drug resistance is a major problem in ovarian cancer. Triggering apoptosis using death ligands such as tumour necrosis factor-related apoptosis inducing ligand (TRAIL) might overcome chemoresistance.
Methods:
We investigated whether acquired cisplatin resistance affects sensitivity to recombinant human (rh) TRAIL alone or in combination with cisplatin in an ovarian cancer cell line model consisting of A2780 and its cisplatin-resistant subline CP70.
Results:
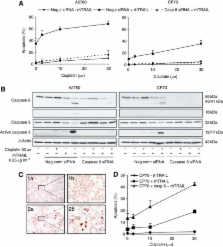
Combining cisplatin and rhTRAIL strongly enhanced apoptosis in both cell lines. CP70 expressed less caspase 8 protein, whereas mRNA levels were similar compared with A2780. Pre-exposure of particularly CP70 to cisplatin resulted in strongly elevated caspase 8 protein and mRNA levels. Caspase 8 mRNA turnover and protein stability in the presence or absence of cisplatin did not differ between both cell lines. Cisplatin-induced caspase 8 protein levels were essential for the rhTRAIL-sensitising effect as demonstrated using caspase 8 small-interfering RNA (siRNA) and caspase-8 overexpressing constructs. Cellular FLICE-inhibitory protein (c-FLIP) and p53 siRNA experiments showed that neither an altered caspase 8/c-FLIP ratio nor a p53-dependent increase in DR5 membrane expression following cisplatin were involved in rhTRAIL sensitisation.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis.
- Record: found
- Abstract: found
- Article: not found
A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
- Record: found
- Abstract: found
- Article: not found
