- Record: found
- Abstract: found
- Article: found
A Granulin-Like Growth Factor Secreted by the Carcinogenic Liver Fluke, Opisthorchis viverrini, Promotes Proliferation of Host Cells
Read this article at
Abstract
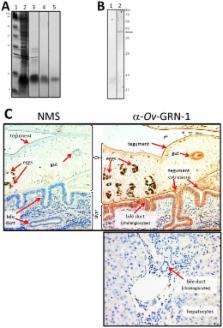
The human liver fluke, Opisthorchis viverrini, infects millions of people throughout south-east Asia and is a major cause of cholangiocarcinoma, or cancer of the bile ducts. The mechanisms by which chronic infection with O. viverrini results in cholangiocarcinogenesis are multi-factorial, but one such mechanism is the secretion of parasite proteins with mitogenic properties into the bile ducts, driving cell proliferation and creating a tumorigenic environment. Using a proteomic approach, we identified a homologue of human granulin, a potent growth factor involved in cell proliferation and wound healing, in the excretory/secretory (ES) products of the parasite. O. viverrini granulin, termed Ov-GRN-1, was expressed in most parasite tissues, particularly the gut and tegument. Furthermore, Ov-GRN-1 was detected in situ on the surface of biliary epithelial cells of hamsters experimentally infected with O. viverrini. Recombinant Ov-GRN-1 was expressed in E. coli and refolded from inclusion bodies. Refolded protein stimulated proliferation of murine fibroblasts at nanomolar concentrations, and proliferation was inhibited by the MAPK kinase inhibitor, U0126. Antibodies raised to recombinant Ov-GRN-1 inhibited the ability of O. viverrini ES products to induce proliferation of murine fibroblasts and a human cholangiocarcinoma cell line in vitro, indicating that Ov-GRN-1 is the major growth factor present in O. viverrini ES products. This is the first report of a secreted growth factor from a parasitic worm that induces proliferation of host cells, and supports a role for this fluke protein in establishment of a tumorigenic environment that may ultimately manifest as cholangiocarcinoma.
Author Summary
The oriental liver fluke is endemic through South-East Asia and is the major cause of cause of liver cancer in north-eastern Thailand. The molecules that are secreted by the parasite cause cells to multiply quicker than they normally would, and excessive cell growth is a key stage in the initiation of many cancers. We identified a secreted protein from the fluke, termed granulin, which has a similar structure to a human growth factor associated with many aggressive cancers. Granulin is secreted by the parasite into the bile ducts where it causes host cells to proliferate. The proliferative activity of fluke secreted proteins was blocked by antibodies against granulin, indicating that it is the major cell growth-inducing molecule released by the parasite. Identifying the function of granulin will enable us to understand how and why this debilitating yet neglected pathogen causes cancer in so many people in South-East Asia. This and future work will contribute towards the development of new strategies to reduce both parasite prevalence and the incidence of the most fatal of liver cancers in Thailand.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity.
- Record: found
- Abstract: found
- Article: not found
Progranulin is a mediator of the wound response.
- Record: found
- Abstract: found
- Article: not found