- Record: found
- Abstract: found
- Article: found
Brain sugar consumption during neuronal activation detected by CEST functional MRI at ultra-high magnetic fields
Read this article at
Abstract
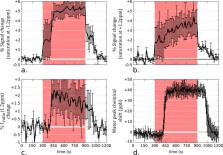
Blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) indirectly measures brain activity based on neurovascular coupling, a reporter that limits both the spatial and temporal resolution of the technique as well as the cellular and metabolic specificity. Emerging methods using functional spectroscopy (fMRS) and diffusion-weighted fMRI suggest that metabolic and structural modifications are also taking place in the activated cells. This paper explores an alternative metabolic imaging approach based on Chemical Exchange Saturation Transfer (CEST) to assess potential metabolic changes induced by neuronal stimulation in rat brains at 17.2 T. An optimized CEST-fMRI data acquisition and processing protocol was developed and used to experimentally assess the feasibility of glucoCEST-based fMRI. Images acquired under glucose-sensitizing conditions showed a substantial negative contrast that highlighted the same brain regions as those activated with BOLD-fMRI. We ascribe this novel fMRI contrast to CEST’s ability to monitor changes in the local concentration of glucose, a metabolite closely coupled to neuronal activity. Our findings are in good agreement with literature employing other modalities. The use of CEST-based techniques for fMRI is not limited to glucose detection; other metabolic pathways involved in neuronal activation could be potentially probed. Moreover, being non invasive, it is conceivable that the same approach can be used for human studies.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI.
- Record: found
- Abstract: not found
- Article: not found
Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging.
- Record: found
- Abstract: found
- Article: not found