- Record: found
- Abstract: found
- Article: found
Prenatal immune programming of the sex-dependent risk for major depression
Read this article at
Abstract
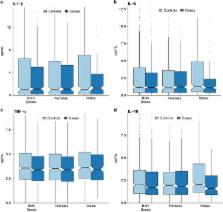
Maternal immune functioning during pregnancy contributes to sex-dependent deficits in neurodevelopment and to behaviors associated with affective traits in preclinical studies, and has been indirectly associated with offspring depression in epidemiologic studies. We therefore investigated the association between immune activity during pregnancy and the risk of depression among male and female offspring. We conducted a case–control study of depression ( n=484 cases and n=774 controls) using data from the New England Family Study, a pregnancy cohort enrolled between 1959 and 1966 that assessed psychiatric outcomes in adult offspring (mean age=39.7 years). We assayed concentrations of three pro-inflammatory cytokines, interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, and the anti-inflammatory cytokine, IL-10, in maternal serum collected at the end of the second and beginning of the third trimesters. High maternal TNF-α was associated with reduced odds of depression among both male and female offspring (odds ratio (OR)=0.68; confidence interval (CI)=0.48, 0.98). However, when considering the TNF-α to IL-10 ratio, a measure of the ratio of pro- to anti-inflammatory loading, maternal immune effects on offspring depression differed significantly by sex ( χ 2=13.9, degrees of freedom=4, P=0.008). Among females, higher maternal TNF-α:IL-10 was associated with reduced odds of depression (OR=0.51; CI=0.32, 0.81), whereas, among males, high maternal TNF-α:IL-10 was associated with elevated odds of depression (OR=1.86; CI=1.02, 3.39). Thus, the balance between TNF-α and IL-10 in maternal prenatal serum was associated with depression in a sex-dependent manner. These findings are consistent with the role of TNF-α in the maturation of the sexually dimorphic fetal brain circuitry that regulates stress and affective responses, and support a prenatal stress-immune model of depression pathogenesis.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Effects of prenatal infection on brain development and behavior: a review of findings from animal models.
- Record: found
- Abstract: found
- Article: not found
Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging.
- Record: found
- Abstract: found
- Article: not found