- Record: found
- Abstract: found
- Article: found
High Fat Intake Leads to Acute Postprandial Exposure to Circulating Endotoxin in Type 2 Diabetic Subjects
Read this article at
Abstract
OBJECTIVE
To evaluate the changes in circulating endotoxin after a high–saturated fat meal to determine whether these effects depend on metabolic disease state.
RESEARCH DESIGN AND METHODS
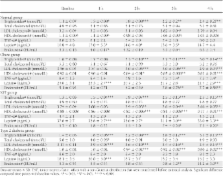
Subjects ( n = 54) were given a high-fat meal (75 g fat, 5 g carbohydrate, 6 g protein) after an overnight fast (nonobese control [NOC]: age 39.9 ± 11.8 years [mean ± SD], BMI 24.9 ± 3.2 kg/m 2, n = 9; obese: age 43.8 ± 9.5 years, BMI 33.3 ± 2.5 kg/m 2, n = 15; impaired glucose tolerance [IGT]: age 41.7 ± 11.3 years, BMI 32.0 ± 4.5 kg/m 2, n = 12; type 2 diabetic: age 45.4 ± 10.1 years, BMI 30.3 ± 4.5 kg/m 2, n = 18). Blood was collected before (0 h) and after the meal (1–4 h) for analysis.
RESULTS
Baseline endotoxin was significantly higher in the type 2 diabetic and IGT subjects than in NOC subjects, with baseline circulating endotoxin levels 60.6% higher in type 2 diabetic subjects than in NOC subjects ( P < 0.05). Ingestion of a high-fat meal led to a significant rise in endotoxin levels in type 2 diabetic, IGT, and obese subjects over the 4-h time period ( P < 0.05). These findings also showed that, at 4 h after a meal, type 2 diabetic subjects had higher circulating endotoxin levels (125.4%↑) than NOC subjects ( P < 0.05).
CONCLUSIONS
These studies have highlighted that exposure to a high-fat meal elevates circulating endotoxin irrespective of metabolic state, as early as 1 h after a meal. However, this increase is substantial in IGT and type 2 diabetic subjects, suggesting that metabolic endotoxinemia is exacerbated after high fat intake. In conclusion, our data suggest that, in a compromised metabolic state such as type 2 diabetes, a continual snacking routine will cumulatively promote their condition more rapidly than in other individuals because of the greater exposure to endotoxin.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Metabolic endotoxemia initiates obesity and insulin resistance.
- Record: found
- Abstract: found
- Article: not found
Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia.
- Record: found
- Abstract: found
- Article: not found