- Record: found
- Abstract: found
- Article: found
Nanoparticle Delivery of miR-34a Eradicates Long-term-cultured Breast Cancer Stem Cells via Targeting C22ORF28 Directly
Read this article at
Abstract
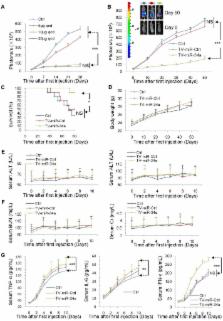
Rationale: Cancer stem cells (CSCs) have been implicated as the seeds of therapeutic resistance and metastasis, due to their unique abilities of self-renew, wide differentiation potentials and resistance to most conventional therapies. It is a proactive strategy for cancer therapy to eradicate CSCs. Methods: Tumor tissue-derived breast CSCs (BCSC), including XM322 and XM607, were isolated by fluorescence-activated cell sorting (FACS); while cell line-derived BCSC, including MDA-MB-231.SC and MCF-7.SC, were purified by magnetic-activated cell sorting (MACS). Analyses of microRNA and mRNA expression array profiles were performed in multiple breast cell lines. The mentioned nanoparticles were constructed following the standard molecular cloning protocol. Tissue microarray analysis has been used to study 217 cases of clinical breast cancer specimens. Results: Here, we have successfully established four long-term maintenance BCSC that retain their tumor-initiating biological properties. Our analyses of microarray and qRT-PCR explored that miR-34a is the most pronounced microRNA for investigation of BCSC. We establish hTERT promoter-driven VISA delivery of miR-34a (TV-miR-34a) plasmid that can induce high throughput of miR-34a expression in BCSC. TV-miR-34a significantly inhibited the tumor-initiating properties of long-term-cultured BCSC in vitro and reduced the proliferation of BCSC in vivo by an efficient and safe way. TV-miR-34a synergizes with docetaxel, a standard therapy for invasive breast cancer, to act as a BCSC inhibitor. Further mechanistic investigation indicates that TV-miR-34a directly prevents C22ORF28 accumulation, which abrogates clonogenicity and tumor growth and correlates with low miR-34 and high C22ORF28 levels in breast cancer patients. Conclusion: Taken together, we generated four long-term maintenance BCSC derived from either clinical specimens or cell lines, which would be greatly beneficial to the research progress in breast cancer patients. We further developed the non-viral TV-miR-34a plasmid, which has a great potential to be applied as a clinical application for breast cancer therapy.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Evolution of the cancer stem cell model.
- Record: found
- Abstract: found
- Article: not found
Lipid Nanoparticle Systems for Enabling Gene Therapies.
- Record: found
- Abstract: found
- Article: not found