- Record: found
- Abstract: found
- Article: found
Catalpol promotes articular cartilage repair by enhancing the recruitment of endogenous mesenchymal stem cells
Read this article at
Abstract
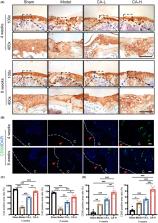
Articular cartilage defect is challenged by insufficient regenerative ability of cartilage. Catalpol (CA), the primary active component of Rehmanniae Radix, could exert protective effects against various diseases. However, the impact of CA on the treatment of articular cartilage injuries is still unclear. In this study, full‐thickness articular cartilage defect was induced in a mouse model via surgery. The animals were intraperitoneally injected with CA for 4 or 8 weeks. According to the results of macroscopic observation, micro‐computed tomography CT (μCT), histological and immunohistochemistry staining, CA treatment could promote mouse cartilage repair, resulting in cartilage regeneration, bone structure improvement and matrix anabolism. Specifically, an increase in the expression of CD90, the marker of mesenchymal stem cells (MSCs), in the cartilage was observed. In addition, we evaluated the migratory and chondrogenic effects of CA on MSCs. Different concentration of CA was added to C3H10 T1/2 cells. The results showed that CA enhanced cell migration and chondrogenesis without affecting proliferation. Collectively, our findings indicate that CA may be effective for the treatment of cartilage defects via stimulation of endogenous MSCs.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse.
- Record: found
- Abstract: found
- Article: not found
International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture.
- Record: found
- Abstract: found
- Article: not found