- Record: found
- Abstract: found
- Article: found
A retrospective evaluation of the efficacy of intravenous bumetanide and comparison of potency with furosemide
Read this article at
Abstract
Background
The potency of intravenous bumetanide to furosemide using a ratio of 1:40 has been suggested; however, there are little data supporting this ratio. Recent drug shortages required the use of bumetanide in a large patient population, enabling further characterization of the efficacy of IV bumetanide.
Objective
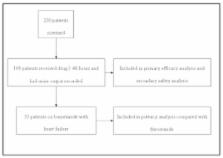
The primary objective of this study was to estimate a dose-response effect of IV bumetanide on urine output (UOP) in all patients that received 48 hours of therapy as well as in a subgroup of patients with heart failure (HF). This subgroup was used to compare the potency of bumetanide with furosemide. A secondary safety objective described electrolyte replacement required during therapy.
Methods
This was a single-center retrospective study examining the dose-response effect of IV bumetanide in patients receiving at least 48 hours of intermittent (iIV) or continuous (cIV) dosing, measured by UOP per mg of drug received (mL/mg). The potency of IV bumetanide was compared with furosemide in a subset of patients with HF using pre-existing data. The safety of IV bumetanide was analyzed by quantifying electrolyte replacement received during the study period.
Results
The primary outcome was higher in the iIV group (n=93) at 1273 ± 844 mL/mg compared with the cIV group (n=16) at 749 ± 370 mL/mg (P=0.002). Among patients with HF who received furosemide (iIV n=30, cIV n=26) or bumetanide (iIV n=30, cIV n=3), a potency ratio of 41:1 was found for the iIV group and 34:1 for all patients with HF. There was no significant difference in electrolyte replacement between groups.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Diuretic strategies in patients with acute decompensated heart failure.
- Record: found
- Abstract: not found
- Article: not found
ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association.
- Record: found
- Abstract: not found
- Article: not found