- Record: found
- Abstract: found
- Article: found
Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non–Small-Cell Lung Cancer After Previous Systemic Treatment Failure—A Retrospective Study
Read this article at
Abstract
Background
Pre-clinical and clinical evidences support that simultaneous blockade of programmed death-1 (PD-1) and vascular endothelial growth factor receptor (VEGFR) can enhance antigen-specific T-cell migration, and show tolerable toxicity with favorable antitumor activity in patients. In this study, we aimed to assess the safety and efficacy of anlotinib, a novel multitarget tyrosine kinase inhibitor for VEGFR, platelet-derived growth receptor (PDGFR), and the stem cell-factor receptor (c-Kit), combined with anti-PD-1 treatment in patients with advanced NSCLC.
Methods
Sixty-seven patients with previously treated advanced NSCLC receiving anti-PD-1 agents concomitant with anlotinib were retrospectively enrolled in an IRB approved study. Anti-PD-1 agents including pembrolizumab, nivolumab, camrelizumab, toripalimab, sintilimab, and tislelizumab were administered every two or three weeks until disease progression or unacceptable toxicity was reached. Anlotinib was administered orally once daily on days 1–14 of a 21-day cycle. The safety and tolerability of the combination treatment were assessed by the incidence of adverse events. The efficacy of the treatment was assessed by the tumor response and survival.
Results
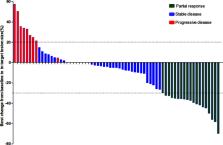
With a median follow-up period of 8.7 months, treatment-related adverse events occurred in 85% (57/67) of patients and grade 3–4 adverse events were observed in 27 patients (40%). No unexpected adverse events or significantly increased toxicities were observed. Complete response was not observed, 19 patients had partial response (28.4%), 39 had stable disease (58.2%) and 9 had progressive disease (13.4%). The overall response (ORR) and disease control rates (DCR) were 28.4% and 86.6%, respectively. The median progression-free survival (PFS) was 6.9 months (95% CI, 5.5-8.3 months) and overall survival (OS) was 14.5 months (95% CI, 10.9-18.1 months). The benefit of anti-PD-1 plus anlotinib was also observed in patients with EGFR mutation positive, liver metastases and brain metastases.
Conclusion
Anti-PD-1 treatment concomitant with anlotinib has tolerable toxicity and favorable antitumor activity in patients with previously treated advanced NSCLC. Our results add to the growing evidence that supports the benefits of combining immunotherapy with antiangiogenic drugs. This combination could be further evaluated with or without chemotherapy, since no additional toxicity was observed in the combination treatment.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found
Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found