- Record: found
- Abstract: found
- Article: found
Electrochemical Study of Iodide in the Presence of Phenol and o-Cresol: Application to the Catalytic Determination of Phenol and o-Cresol
Read this article at
Abstract
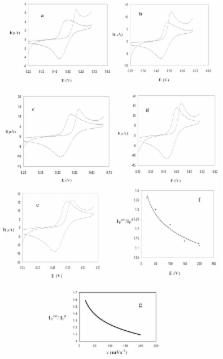
The electrochemical oxidation of iodide in the presence of phenol and o-cresol was investigated at a glassy carbon electrode in buffered media by cyclic voltammetry, linear sweep voltammetry and controlled–potential coulometry. The experimental results indicate that the phenol and o-cresol convert to their derivatives after participating in a halogenation coupled reaction (quasi-catalytic reaction) following the oxidation of iodide to iodine. The concentrations of phenol and o-cresol have been determined in aqueous solutions according to the linear dependence of quasi-catalytic peak currents with the concentration. The calibration graphs show two linear sections of 0.0 to 1.0×10 -4 M and 2.0×10 -4 to 1.0 ×10 -3 M for phenol and 4.2×10 -5 to 1.0×10 -4 M and 2.0×10 -4 to 1.0×10 -3 M for o-cresol. The theoretical detection limits and the relative standard deviations for ten measurements of phenol and o-cresol are 1.125×10 -5 M, 1.06% and 4.201×10 -5 M, 1.44%, respectively.
Related collections
Most cited references39
- Record: found
- Abstract: not found
- Article: not found
Carbon paste electrodes modified with enzymes, tissues, and cells
- Record: found
- Abstract: not found
- Article: not found
Chemically modified carbon paste electrodes in voltammetric analysis
- Record: found
- Abstract: found
- Article: not found