- Record: found
- Abstract: found
- Article: found
In Situ Glycan Analysis and Editing in Living Systems

Read this article at
Abstract
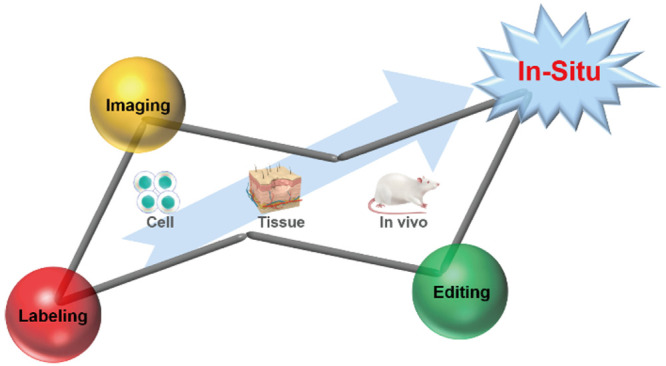
Besides proteins and nucleic acids, carbohydrates are also ubiquitous building blocks of living systems. Approximately 70% of mammalian proteins are glycosylated. Glycans not only provide structural support for living systems but also act as crucial regulators of cellular functions. As a result, they are considered essential pieces of the life science puzzle. However, research on glycans has lagged far behind that on proteins and nucleic acids. The main reason is that glycans are not direct products of gene coding, and their synthesis is nontemplated. In addition, the diversity of monosaccharide species and their linkage patterns contribute to the complexity of the glycan structures, which is the molecular basis for their diverse functions. Research in glycobiology is extremely challenging, especially for the in situ elucidation of glycan structures and functions. There is an urgent need to develop highly specific glycan labeling tools and imaging methods and devise glycan editing strategies. This Perspective focuses on the challenges of in situ analysis of glycans in living systems at three spatial levels (i.e., cell, tissue, and in vivo) and highlights recent advances and directions in glycan labeling, imaging, and editing tools. We believe that examining the current development landscape and the existing bottlenecks can drive the evolution of in situ glycan analysis and intervention strategies and provide glycan-based insights for clinical diagnosis and therapeutics.
Related collections
Most cited references205
- Record: found
- Abstract: found
- Article: not found
Glycosylation in cellular mechanisms of health and disease.
- Record: found
- Abstract: found
- Article: not found
Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation.
- Record: found
- Abstract: found
- Article: not found