- Record: found
- Abstract: found
- Article: found
Analysis of the distribution of vagal afferent projections from different peripheral organs to the nucleus of the solitary tract in rats

Read this article at
Abstract
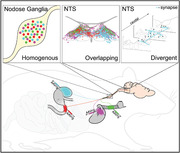
Anatomical tracing studies examining the vagal system can conflate details of sensory afferent and motor efferent neurons. Here, we used a serotype of adeno‐associated virus that transports retrogradely and exhibits selective tropism for vagal afferents, to map their soma location and central termination sites within the nucleus of the solitary tract (NTS). We examined the vagal sensory afferents innervating the trachea, duodenum, stomach, or heart, and in some animals, from two organs concurrently. We observed no obvious somatotopy in the somata distribution within the nodose ganglion. The central termination patterns of afferents from different organs within the NTS overlap substantially. Convergence of vagal afferent inputs from different organs onto single NTS neurons is observed. Abdominal and thoracic afferents terminate throughout the NTS, including in the rostral NTS, where the 7th cranial nerve inputs are known to synapse. To address whether the axonal labeling produced by viral transduction is so widespread because it fills axons traveling to their targets, and not just terminal fields, we labeled pre and postsynaptic elements of vagal afferents in the NTS . Vagal afferents form multiple putative synapses as they course through the NTS, with each vagal afferent neuron distributing sensory signals to multiple second‐order NTS neurons. We observe little selectivity between vagal afferents from different visceral targets and NTS neurons with common neurochemical phenotypes, with afferents from different organs making close appositions with the same NTS neuron. We conclude that specific viscerosensory information is distributed widely within the NTS and that the coding of this input is probably determined by the intrinsic properties and projections of the second‐order neuron.
Abstract
We injected retrograde pseudotyped adeno‐associated viruses into different peripheral organs and mapped the axonal projections of vagal viscerosensory neurons within the nucleus of the solitary tract (NTS). We observed no obvious viscerotopy, with axons from different organs overlapping in NTS subnuclei and with NTS neurons of different neurochemical phenotype. The implications for deciphering this viscerosensory code are discussed.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging.
- Record: found
- Abstract: found
- Article: not found
A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons
- Record: found
- Abstract: found
- Article: not found