- Record: found
- Abstract: found
- Article: not found
Tenderization of Bovine Longissimus Dorsi Muscle using Aqueous Extract from Sarcodon aspratus
Read this article at
Abstract
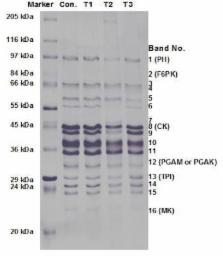
The aim of this study was to investigate the effects of aqueous extract from Sarcodon aspratus on tenderization of the bovine longissimus dorsi muscles in comparison with commercial proteolytic enzymes. Furthermore, meat quality and muscle protein degradation were examined. We marinated meat with 2% Sarcodon aspratus extract, 2% kiwi extract, and 0.2% papain. Beef chunks (3×3×3 cm 3) were marinated with distilled water (control), Sarcodon aspratus extract (T1), kiwi extract (T2) or papain (T3) for 48 h at 4℃. There were no significant differences in muscle pH and lightness between control and treated samples. T1 had the lowest redness ( p<0.01), and higher cooking loss and water holding capacity than control and T2 ( p<0.05). T1 and T3 exhibited lower shear force values than control ( p<0.05). Total protein solubility did not differ significantly between T1 and control, but T1 had less myofibrillar protein solubility than control and T2 ( p<0.001). The degradation of myosin heavy chain in T1 and T3 was observed. This degradation of myofibrillar protein suggests that Sarcodon aspratus extract could influence tenderization. These results show that aqueous extract of Sarcodon aspratus extract actively affect the tenderness of the bovine longissimus dorsi muscle.
Related collections
Most cited references30
- Record: found
- Abstract: not found
- Article: not found
Determination of serum proteins by means of the biuret reaction.
- Record: found
- Abstract: found
- Article: not found
The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle.
- Record: found
- Abstract: found
- Article: not found
