- Record: found
- Abstract: found
- Article: found
Association between nucleotide excision repair gene polymorphism and colorectal cancer risk
Read this article at
Abstract
Background
The nucleotide excision repair system removes a wide variety of DNA lesions from the human genome, and plays an important role in maintaining genomic stability. Single nucleotide polymorphisms (SNPs) in nucleotide excision repair are associated with the various forms of tumor susceptibility. However, the relationship between NER polymorphism and colorectal cancer is not clear.
Methods
In this study, three candidate SNPs including ERCC4 (rs6498486), ERCC1 (rs3212986), and ERCC5 (rs17655) were analyzed in 1101colorectal cancer patients and 1175 healthy control patients from Jiangsu province (China). Then, we performed Immunohistochemistry, qPCR, and luciferase assay to determine the potential mechanisms.
Results
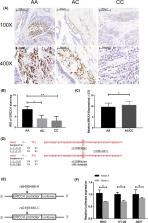
The ERCC4 rs6498486 AC/CC genotypes show lower susceptibility to CRC than those carrying rs6498486 AA (Adjusted OR = 0.82, 95% CI = 0.69‐0.97). However, we did not observe any association between the colorectal cancer risk and the rs3212986(ERCC1) and rs17655(ERCC5) polymorphisms. Immunohistochemistry, qPCR, and luciferase assay revealed that rs6498486 A > C polymorphism in the ERCC4 promoter region could lessen the expression level of ERCC4 by impacting the binding ability of the transcription factor NF‐kB, thereby affecting the transcription activity of the ERCC4 gene and decreased ERCC4 gene expression.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
NF-kappaB signaling in cerebral ischemia.
- Record: found
- Abstract: found
- Article: found
Genetics, Cytogenetics, and Epigenetics of Colorectal Cancer
- Record: found
- Abstract: found
- Article: not found