- Record: found
- Abstract: found
- Article: found
Stem Cells from Human Exfoliated Deciduous Teeth Ameliorate Diabetic Nephropathy In Vivo and In Vitro by Inhibiting Advanced Glycation End Product-Activated Epithelial-Mesenchymal Transition
Read this article at
Abstract
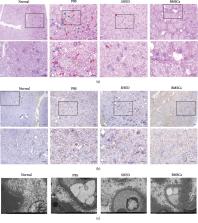
Diabetic nephropathy (DN) is a major cause of chronic kidney disease. It has been proven that mesenchymal stem cells (MSCs) have therapeutic effects on kidney disease. Stem cells from human exfoliated deciduous teeth (SHED) are MSCs that are derived from dental pulps in exfoliated deciduous teeth from young patients and therefore have a high proliferation rate and an easy access. Hence, we aimed to explore the effect of SHED on DN in Goto-Kakizaki (GK) rats. SHED were administered via the tail vein. Blood glucose, serum triglycerides and cholesterol, body weight, and urinary albumin were measured before and after administration. At 8 weeks after administration, real-time PCR, immunohistochemistry (IHC), and electron microscopy were employed to examine pathological changes in glomerular and tubulointerstitial tissue. Kidney weight and serum IL-1, IL-10, TNF- α, TGF- β, and HGF levels were measured. SHED engraftment in the kidneys was detected by transfecting green fluorescence protein (GFP). Type II epithelial-mesenchymal transition (EMT) in the tubule-interstitial and arteriolar regions has been reported to be an important pathological characteristic of DN. This study is the first to apply a transwell system for coculture to explore the effects of MSCs on the EMT of human proximal tubular epithelial (HK-2) cells. The effects of SHED on advanced glycation end product- (AGE-) activated EMT in HK-2 cells were explored by real-time PCR and western blot. At 8 weeks after administration, renal injury, including hyperglycemia, hyperlipidemia, increased urinary albumin excretion, ECM accumulation, and a fractional mesangial area, was dramatically attenuated. The serum levels of IL-1, TNF- α, and TGF- β were significantly downregulated, whereas the serum levels of IL-10 and HGF were upregulated by SHED. GFP expression confirmed the engraftment of SHED in diabetic kidneys. In addition, cocultured SHED inhibited AGE-induced EMT in HK-2 cells. In conclusion, SHED offer a novel potential effective therapeutic approach for ameliorating DN.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future.
- Record: found
- Abstract: found
- Article: not found
Mechanisms of mesenchymal stromal cell immunomodulation.
- Record: found
- Abstract: found
- Article: not found