- Record: found
- Abstract: found
- Article: found
Additional chemotherapy improves survival in stage II–III pulmonary sarcomatoid carcinoma patients undergoing surgery: a propensity scoring matching analysis
Read this article at
Abstract
Background
The role of additional chemotherapy in pulmonary sarcomatoid carcinoma (PSC) is controversial. This study aimed to investigate the function of chemotherapy in PSC patients with surgical resection.
Methods
PSC patient information between 2004 to 2016 was extracted from the Surveillance, Epidemiology, and End Results (SEER) database. X-tile software was used to calculate the optimal cut-off value to divide groups. The disease stages were recalculated according to the American Joint Commission on Cancer (AJCC) 8 th edition tumor-node-metastasis (TNM) staging system. Propensity score matching (PSM) analysis was conducted to balance the baseline of patients. Kaplan-Meier analysis and Cox proportional hazards analysis were used to evaluate survival outcome.
Results
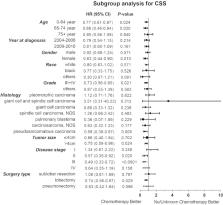
A total of 865 PSC patients were included in our study. Among them, 611 patients were only operated with surgery, and the 254 others were treated with additional chemotherapy. The median age was 69.0 years (interquartile range, 61.6 to 76.3 years). Kaplan-Meier analysis showed that patients with additional chemotherapy had longer overall survival (OS) and cancer-specific survival (CSS, P<0.05). The median OS and the 1-, 3-, 5-year OS rates were 36.0 months (95% CI: 20.5–51.5 months), 72.7%, 49.6% and 38.5% in the chemotherapy group and 29.0 months (95% CI: 23.6–34.4 months), 63.2%, 44.5% and 37.6% in the non-chemotherapy group, respectively. The OS advantage of chemotherapy was not statistically significant after PSM analysis. Moreover, Cox proportional hazards model showed that chemotherapy was an independent prognosis factor for better OS and CSS. In subgroup of stages II and III, the chemotherapy group had a survival advantage (P<0.05). Patients with young age, female gender, low histology grade, large tumor size and lobectomy surgical resection benefited more from chemotherapy.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer.
- Record: found
- Abstract: found
- Article: not found
The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification.
- Record: found
- Abstract: not found
- Article: not found