- Record: found
- Abstract: found
- Article: found
Insulin-Mimicking Bioactivities of Acylated Inositol Glycans in Several Mouse Models of Diabetes with or without Obesity
Read this article at
Abstract
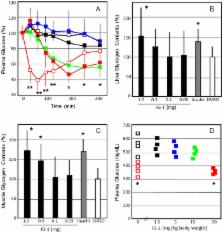
Insulin-mimetic species of low molecular weight are speculated to mediate some intracellular insulin actions. These inositol glycans, which are generated upon insulin stimulation from glycosylphosphatidylinositols, might control the activity of a multitude of insulin effector enzymes. Acylated inositol glycans (AIGs) are generated by cleavage of protein-free GPI precursors through the action of GPI-specific phospholipase C (GPI-PLC) and D (GPI-PLD). We synthesized AIGs (IG-1, IG-2, IG-13, IG-14, and IG-15) and then evaluated their insulin-mimicking bioactivities. IG-1 significantly stimulated glycogen synthesis and lipogenesis in 3T3-L1 adipocytes and rat isolated adipocytes dose-dependently. IG-2 significantly stimulated lipogenesis in rat isolated adipocytes dose-dependently. IG-15 also enhanced glycogen synthesis and lipogenesis in 3T3-L1 adipocytes. The administration of IG-1 decreased plasma glucose, increased glycogen content in liver and skeletal muscles and improved glucose tolerance in C57B6N mice with normal diets. The administration of IG-1 decreased plasma glucose in STZ-diabetic C57B6N mice. The treatment of IG-1 decreased plasma glucose, increased glycogen content in liver and skeletal muscles and improved glucose tolerance in C57B6N mice with high fat-diets and db/db mice. The long-term treatment of IG-1 decreased plasma glucose and reduced food intake and body weight in C57B6N mice with high fat-diets and ob/ob mice. Thus, IG-1 has insulin-mimicking bioactivities and improves glucose tolerance in mice models of diabetes with or without obesity.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Phosphorylation of IRS proteins, insulin action, and insulin resistance.
- Record: found
- Abstract: found
- Article: not found
The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research.
- Record: found
- Abstract: found
- Article: not found