- Record: found
- Abstract: found
- Article: found
Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway
Read this article at
Abstract
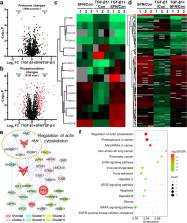
Breast cancer metastasis is the main cause of cancer death in women, so far, no effective treatment has inhibited breast cancer metastasis. Sulforaphane (SFN), a natural compound derived from broccoli, has shown potential health benefits in many cancers. However, research on breast cancer metastasis is still insufficient. Here, we showed that SFN, including its two isomers of R-SFN and S-SFN, significantly inhibited TGF-β1-induced migration and invasion in breast cancer cells. Proteomic and phosphoproteomic analysis showed that SFN affected the formation of the cytoskeleton. Subsequent experiments confirmed that SFN significantly inhibited TGF-β1-induced actin stress fiber formation and the expression of actin stress fiber formation-associated proteins, including paxillin, IQGAP1, FAK, PAK2, and ROCK. Additionally, SFN is directly bound to RAF family proteins (including ARAF, BRAF, and CRAF) and inhibited MEK and ERK phosphorylation. These in vitro results indicate that SFN targets the RAF/MEK/ERK signaling pathway to inhibit the formation of actin stress fibers, thereby inhibiting breast cancer cell metastasis.
Related collections
Most cited references75

- Record: found
- Abstract: found
- Article: found
The PRIDE database and related tools and resources in 2019: improving support for quantification data
- Record: found
- Abstract: found
- Article: not found
Roles of TGFbeta in metastasis.
- Record: found
- Abstract: found
- Article: not found