- Record: found
- Abstract: found
- Article: found
The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction
Read this article at
Abstract
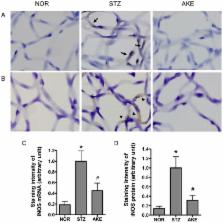
Retinal capillary cell loss is a hallmark of early diabetic retinal changes. Advanced glycation end products (AGEs) are believed to contribute to retinal microvascular cell loss in diabetic retinopathy. In this study, the protective effects of Aster koraiensis extract (AKE) against damage to retinal vascular cells were investigated in streptozotocin (STZ)-induced diabetic rats. To examine this issue further, AGE accumulation, nuclear factor-kappaB (NF-κB) and inducible nitric oxide synthase (iNOS) were investigated using retinal trypsin digests from streptozotocin-induced diabetic rats. In the diabetic rats, TUNEL (Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling)-positive retinal microvascular cells were markedly increased. Immunohistochemical studies revealed that AGEs were accumulated within the retinal microvascular cells, and this accumulation paralleled the activation of NF-κB and the expression of iNOS in the diabetic rats. However, AKE prevented retinal microvascular cell apoptosis through the inhibition of AGE accumulation and NF-κB activation. Moreover, to determine the active compounds of AKE, two major compounds, chlorogenic acid and 3,5-di- O-caffeoylquinic acid, were tested in an in vitro assay. Among these compounds, chlorogenic acid significantly reduced AGE formation as well as AGE/RAGE (receptor for AGEs) binding activity. These results suggest that AKE, particularly chlorogenic acid, is useful in inhibiting AGE accumulation in retinal vessels and exerts a preventive effect against the injuries of diabetic retinal vascular cells.
Related collections
Most cited references58
- Record: found
- Abstract: not found
- Article: not found
Nitric oxide synthases: roles, tolls, and controls.
- Record: found
- Abstract: found
- Article: not found
Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose.
- Record: found
- Abstract: found
- Article: not found