- Record: found
- Abstract: found
- Article: found
HPV Testing by cobas HPV Test in a Population from Catalonia
Read this article at
Abstract
Background
HPV testing in cervical cancer screening has been proposed as an alternative or complementary to cytology in women older than 30 years. However, adequate clinical sensitivity and specificity are crucial for a new test to be implemented. Hybrid Capture 2 (HC2) has proved good clinical performance in selecting women at risk for high-grade intraepithelial lesions with a high sensitivity and specificity. cobas HPV Test has been recently launched and its performance in different clinical settings needs to be determined.
Objectives
The aim of this study was to evaluate the cobas HPV Test for the detection of cervical HPV infection in a population of women in Catalonia (Spain) using HC2 as a reference.
Materials and Methods
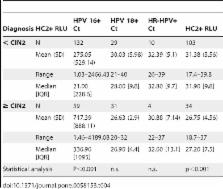
Cervical liquid cytology samples from 958 women have been studied. Sensitivity was analyzed in 60 samples from patients with a high-grade intraepithelial lesion (≥CIN2) on histology and specificity was determined in 898 samples from women with no ≥CIN2. All cases had HC2 and cobas HPV Test performed. Statistical analyses of sensitivity, specificity and comparison between HC2 and cobas HPV Test by a non-inferiority test were applied.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Overview of the European and North American studies on HPV testing in primary cervical cancer screening.
- Record: found
- Abstract: found
- Article: not found
