- Record: found
- Abstract: found
- Article: found
RLN3/RXFP3 Signaling in the PVN Inhibits Magnocellular Neurons via M-like Current Activation and Contributes to Binge Eating Behavior
Read this article at
Abstract
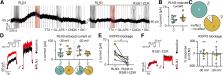
Binge-eating disorder is the most common eating disorder. Various neuropeptides play important roles in the regulation of feeding behavior, including relaxin-3 (RLN3), which stimulates food intake in rats through the activation of the relaxin-family peptide-3 receptor (RXFP3). Here we demonstrate that a likely mechanism underlying the orexigenic action of RLN3 is RXFP3-mediated inhibition of oxytocin- and arginine-vasopressin-synthesizing paraventricular nucleus (PVN) magnocellular neurosecretory cells. Moreover, we reveal that, in male and female rats, this action depends on M-like potassium conductance. Notably, higher intra- and peri-PVN RLN3 fiber densities were observed in females, which may constitute an anatomic substrate for observed sex differences in binge-eating disorder. Finally, in a model of binge-eating in female rats, RXFP3 blockade within the PVN prevented binge-eating behavior. These data demonstrate a direct RLN3/RXFP3 action in the PVN of male and female rats, identify the associated ionic mechanisms, and reveal that hypothalamic RLN3/RXFP3 signaling regulates binge-eating behavior.
SIGNIFICANCE STATEMENT Binge-eating disorder is the most common eating disorder worldwide, affecting women twice as frequently as men. Various neuropeptides play important roles in the regulation of feeding behavior, including relaxin-3, which acts via the relaxin-family peptide-3 receptor (RXFP3). Using a model of binge-eating, we demonstrated that relaxin-3/RXFP3 signaling in the hypothalamic paraventricular nucleus (PVN) is necessary for the expression of binge-eating behavior in female rats. Moreover, we elucidated the neuronal mechanism of RLN3/RXFP3 signaling in PVN in male and female rats and characterized sex differences in the RLN3 innervation of the PVN. These findings increase our understanding of the brain circuits and neurotransmitters involved in binge-eating disorder pathology and identify RXFP3 as a therapeutic target for binge-like eating disorders.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Pathways modulating neural KCNQ/M (Kv7) potassium channels.
- Record: found
- Abstract: found
- Article: not found
Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior.
- Record: found
- Abstract: found
- Article: not found