- Record: found
- Abstract: found
- Article: found
High IL-1α Production Was Induced in the WBN/Kob- Lepr fa Type 2 Diabetes Mellitus Rat Model and Inhibited by Syphacia Muris Infection
Read this article at
Summary
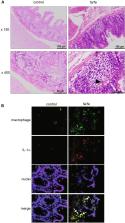
The novel WBN/Kob- Lepr fa ( fa/fa) congenic rat strain is considered a useful rat model of type 2 diabetes mellitus (T2DM). Accumulating findings suggest that low-grade inflammation is a causative factor in T2DM and that circulating levels of inflammatory cytokines are associated with insulin resistance. However, inflammatory cytokine profiles and their correlations with T2DM development/ progression in fa/fa rats have not been studied. In this study, we found that the fa/fa rats had considerably high plasma levels of interleukin (IL)-1α. Abundant cecal IL-1α mRNA expression and cecal inflammation with infiltrating IL-1α-producing macrophages was observed in fa/fa rats. Bone marrow derived macrophages from fa/fa rats expressed high levels of IL-1α upon lipopolysaccharide stimulation. Furthermore, Syphacia muris infection, which delays the onset of T2DM, reduced both plasma and cecal IL-1α levels in fa/fa rats. These results suggest that macrophage infiltration and IL-1α secretion comprise an important part of T2DM development and that S. muris infection inhibits pro-inflammatory cytokine expression in fa/fa rats.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets.
- Record: found
- Abstract: found
- Article: not found
Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes.
- Record: found
- Abstract: found
- Article: not found