- Record: found
- Abstract: found
- Article: found
Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study
Read this article at
Abstract
Background
Despite long-standing clinical use of sodium polystyrene sulphonate (SPS) for hyperkalaemia management in chronic kidney disease (CKD), its safety profile remains poorly investigated.
Methods
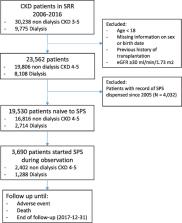
We undertook an observational analysis of nephrology-referred adults with incident CKD Stage 4+ in Sweden during 2006–16 and with no previous SPS use. We studied patterns of use and adverse events associated to SPS initiation during follow-up. Patterns of SPS use were defined by chronicity of treatment and by prescribed dose. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) associated with SPS initiation (time-varying exposure) for the risk of severe (intestinal ischaemia, thrombosis or ulceration/perforation) and minor (de novo dispensation of laxatives or anti-diarrheal drugs) gastrointestinal (GI) events.
Results
Of 19 530 SPS-naïve patients with CKD, 3690 initiated SPS during follow-up. A total of 59% took SPS chronically, with an average of three dispensations/year. The majority (85%) were prescribed lower dosages than specified on the product label. During follow-up, 202 severe and 1149 minor GI events were recorded. SPS initiation was associated with a higher incidence of severe adverse events [adjusted HR 1.25 95% CI 1.05–1.49)], particularly in those receiving per label doses [1.54 (1.09–2.17)] and mainly attributed to ulcers and perforations. SPS initiation was also associated with higher incidence of minor GI events [adjusted HR 1.11 (95% CI 1.03–1.19)], regardless of dose, and mainly accounted for by de novo dispensation of laxatives.
Related collections
Most cited references30
- Record: found
- Abstract: not found
- Article: not found
KDIGO 2012 Clinical Practice Guidelinefor the Evaluation and Management ofChronic Kidney Disease
- Record: found
- Abstract: found
- Article: not found
Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review.
- Record: found
- Abstract: found
- Article: not found