- Record: found
- Abstract: found
- Article: found
Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism

Read this article at
Abstract
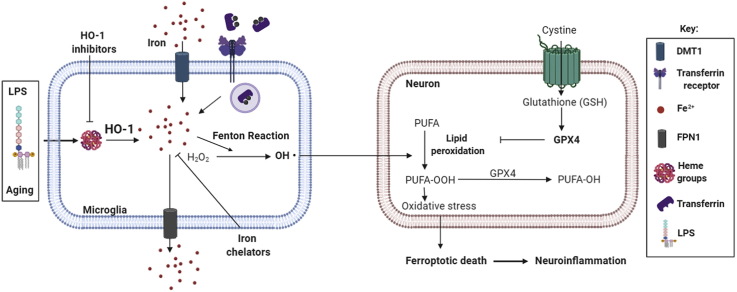
Heme oxygenase-1 (HO-1) is an inducible enzyme known for its anti-inflammatory, antioxidant and neuroprotective effects. However, increased expression of HO-1 during aging and age-related neurodegenerative diseases have been associated to neurotoxic ferric iron deposits. Being microglia responsible for the brain's innate immune response, the aim of this study was to understand the role of microglial HO-1 under inflammatory conditions in aged mice. For this purpose, aged wild type (WT) and LysMCreHmox1 △△ (HMOX1 M-KO) mice that lack HO-1 in microglial cells, were used. Aged WT mice showed higher basal expression levels of microglial HO-1 in the brain than adult mice. This increase was even higher when exposed to an inflammatory stimulus (LPS via i.p.) and was accompanied by alterations in different iron-related metabolism proteins, resulting in an increase of iron deposits, oxidative stress, ferroptosis and cognitive decline. Furthermore, microglia exhibited a primed phenotype and increased levels of inflammatory markers such as iNOS, p65, IL-1β, TNF-α, Caspase-1 and NLRP3. Interestingly, all these alterations were prevented in aged HMOX1 M-KO and WT mice treated with the HO-1 inhibitor ZnPPIX. In order to determine the effects of microglial HO-1-dependent iron overload, aged WT mice were treated with the iron chelator deferoxamine (DFX). DFX caused major improvements in iron, inflammatory and behavioral alterations found in aged mice exposed to LPS. In conclusion, this study highlights how microglial HO-1 overexpression contributes to neurotoxic iron accumulation providing deleterious effects in aged mice exposed to an inflammatory insult.
Graphical abstract
Highlights
-
•
Microglial HO-1 increases with aging and under an acute inflammatory stimulus.
-
•
LPS-dependent microglial HO-1 upregulation during aging leads to iron overload.
-
•
Microglial HO-1-dependent iron accumulation leads to ferroptosis.
-
•
HO-1-dependent iron alterations lead to neuroinflammation.
-
•
HO-1 inhibitors/iron chelators reduce iron accumulation and neuroinflammation.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease
- Record: found
- Abstract: found
- Article: not found
Complement and microglia mediate early synapse loss in Alzheimer mouse models.
- Record: found
- Abstract: found
- Article: not found