- Record: found
- Abstract: found
- Article: found
The very-rapid and the ultra-rapid virologic response to two treatment options in patients with chronic hepatitis C: an interim report of a prospective randomized comparative effectiveness study
Abstract
Background
We aimed in this interim report to compare two registered generic sofosbuvir products for the degree and speed of virologic response to a dual antiviral treatment protocol within the first 2 weeks of treatment.
Methods
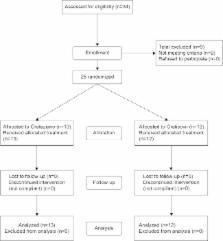
Data collected during the period of this interim report from the first 25 patients randomized to either one of two generic sofosbuvir products (Grateziano or Gratisovir) at a daily dose of one 400 mg tablet plus a weight-based ribavirin dose were analyzed for both the degree and speed of virus load reduction at the end of 1 and 2 weeks from starting treatment.
Results
The baseline Log10 transformed virus load (Log polymerase chain reaction) showed a fairly similar marked and significant reduction in both groups by more than 4 and 5 Logs at the end of week 1 and 2 of starting treatment, respectively. The differences between the two treatment groups at both analysis points were not statistically significant ( P>0.05) by repeated measures factorial analysis of variance test. The differences in proportions of patients with ultra-rapid virologic response at the end of week 1 and very-rapid virologic response at the end of week 2 in both groups were also not statistically significant ( P>0.05).
Video abstract
Most cited references8
- Record: found
- Abstract: found
- Article: not found
A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt.
- Record: found
- Abstract: found
- Article: not found
Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C.
- Record: found
- Abstract: found
- Article: not found