Introduction
Cardiac allograft vasculopathy (CAV) is a major cause of death following cardiac transplant. Because of its diffuse nature, only a few patients warrant treatment with palliative percutaneous coronary intervention.
The use of a fully bioresorbable vascular scaffold (BVS) may potentially reduce long-term cardiovascular events in this subset of patients, especially younger patients with chronic, diffuse disease.
Case Report
A 58-year-old man presented to the emergency department with a 6-month history of exertional shortness of breath (New York Heart Association [NYHA] class III) and chest tightness (Canadian Cardiovascular Society [CCS] class III angina).
He underwent cardiac transplant 20 years previously for sarcoid cardiomyopathy. He presented then with an out-of-hospital cardiac arrest and severe left ventricular systolic dysfunction (ejection fraction 25%). Cardiac biopsy confirmed granulomatous myocarditis consistent with sarcoidosis, with no clinical or radiologic evidence of extracardiac disease. Despite high-dose steroid therapy, he developed refractory ventricular arrhythmias, pulmonary edema, and cardiogenic shock. Given progressive sarcoid cardiomyopathy unresponsive to medical therapy, he was listed for cardiac transplant. In mid-1996 he underwent an orthotopic cardiac transplant. The procedure and postoperative recovery were uneventful. Since then he has been stable with long-term immunosuppressive therapy (prednisolone, cyclosporine, and mycophenolate mofetil), without rejection. The patient returned to his baseline level of function (NYHA class I). Serial screening after transplant, initially with coronary angiography in the first 5 years and subsequently with dobutamine stress echocardiograms, did not reveal CAV.
On this occasion, he presented with NYHA class III heart failure and CCS class III angina without palpitations or syncope. Examination revealed an irregularly irregular pulse of 90 beats per minute, oxygen saturations of 98% for room air, and systemic blood pressure of 118/80 mmHg. General examination revealed evidence of biventricular heart failure. ECG showed atrial fibrillation with a ventricular rate of 89 beats per minute, with an incomplete right bundle branch block but no ischemic changes. Biochemistry tests showed a raised level of high-sensitivity troponin of 38 ng/L (normal <26 ng/L) and an elevated B-type natriuretic peptide level of 1350 ng/L (normal <100 ng/L). Total cholesterol level was 4.3 mmol/L, and low-density lipoprotein level was 2.6 mmol/L with the patient taking pravastatin.
Investigations including cardiac biopsy were negative for cellular or antibody-mediated rejection (grade 0R), and there was no evidence of granulomatous inflammation. Transthoracic echocardiography showed mild left ventricular systolic dysfunction (ejection fraction 45%). Hemodynamic parameters, including pulmonary arterial pressures, were normal on right-sided heart catheterization. Given the newly diagnosed atrial fibrillation, he underwent transesophageal echocardiogram to exclude left atrial appendage thrombus, and was electrically cardioverted to sinus rhythm. Coronary angiography demonstrated a right dominant circulation. There was a discrete critical stenosis (type A lesion) of the midportion of the left anterior descending (LAD) artery due to CAV (Figure 1). There was moderate diffuse disease in the midportion of the left circumflex artery and proximal portion of the right coronary artery.
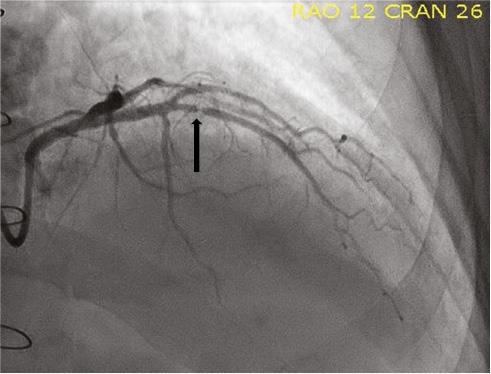
Coronary Angiography Showing a Discrete Critical Stenosis (Black Arrow) of the Midportion of the Left Anterior Descending Artery due to Cardiac Allograft Vasculopathy.
Given his presentation with an acute coronary syndrome and angiographic findings of a critical stenosis of the midportion of the LAD artery, the decision was made to proceed with percutaneous coronary intervention of the midportion of the LAD artery. Given the focal nature of the lesion in the midportion of the LAD artery and the known progressive nature of transplant vasculopathy, it was decided to use a BVS. This would allow the option of future coronary artery bypass grafting. The lesion in the midportion of the LAD artery was predilated with an Emerge 2.5×8 mm semicompliant balloon (Boston Scientific, Natick, MA, USA), inflated at 12 atm. After predilatation there was less than 50% residual stenosis in the midportion of the LAD artery. A 3.0×18 mm Absorb™ everolimus-eluting BVS (Abbott Vascular, Santa Clara, CA, USA) was deployed in the midportion of the LAD artery as per the standard inflation protocol. Postdilation was performed with a 3.25×8 mm noncompliant balloon at 18 atm (Figure 2). Optical coherence tomography (OCT) of the LAD artery confirmed good apposition of the BVS (Figure 3). On discharge, the patient was receiving dual antiplatelet therapy, dabigatran, and amiodarone.

Optical Coherence Tomography of the Left Anterior Descending Artery Showing Good Apposition of the Bioresorbable Vascular Scaffold.
Six weeks later, he represented with symptoms of NYHA class III heart failure and CCS class III angina associated with a troponin level rise of 63 ng/L. He remained in sinus rhythm. Repeated coronary angiography showed a new hazy lesion in the proximal portion of the LAD artery. OCT confirmed a widely patent BVS in the midportion of the LAD artery. There was, however, new plaque rupture in the proximal portion of the LAD artery, away from the previous BVS in the midportion of the LAD artery. The lesion in the proximal portion of the LAD artery was predilated and treated with a Xience Alpine™ everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA). Dabigatran use was ceased, and the patient continued to receive dual antiplatelet therapy. Given his rapidly progressive CAV, he has also been waitlisted for a second cardiac transplant.
Discussion
CAV is one of the major causes of death following cardiac transplant, with a prevalence of 30% at 5 years and 50% at 10 years [1]. It is characterized by diffuse concentric longitudinal intimal hyperplasia in the epicardial coronary arteries. Both immunologic and nonimmunologic risk factors, including cellular and antibody-mediated rejection, donor-specific anti-HLA antibodies, cytomegalovirus infection, and hypercholesterolemia, contribute to the development of CAV [1]. The typical symptoms of myocardial ischemia are absent because of afferent and efferent allograft denervation, and diagnosis can be challenging. Invasive coronary angiography is one method for the surveillance and monitoring of CAV especially in the first 5 years [2]. However, its sensitivity is also affected by the often diffuse, concentric, and longitudinal nature of the disease. Other invasive investigational methods such as intravascular ultrasonography, coronary flow reserve measurements, and OCT can also assist in the diagnosis of CAV. OCT allows high-resolution quantitative evaluation of the coronary artery wall structure and composition, and is particularly useful in detecting early CAV and plaque stability [3].
Treatment of CAV is difficult. Repeated transplant is the only definitive approach. However, this raises ethical issues, including allocation of an already scarce resource of a donor heart for a second transplant when many patients die waiting for their first transplant. Surgical revascularization with coronary artery bypass grafting surgery can be performed for suitable patients, but studies on this subject are limited. Because of its diffuse nature, only a few patients with CAV warrant palliative percutaneous coronary intervention, which is associated with a higher rate of restenosis compared with native coronary artery disease. Drug-eluting stents had lower rates of target lesion revascularization and a lower composite rate of death and nonfatal myocardial infarction compared with bare metal stents in a single-center retrospective study of clinical outcomes of cardiac transplant patients receiving drug-eluting stents versus bare metal stents [4]. There are, however, limitations of drug-eluting stents, such as polymer-associated inflammatory and thrombotic reactions resulting in accelerated neoatherosclerosis, which increases the risk of stent thrombosis, myocardial infarctions, and the requirement for repeated revascularization [5].
Device Description
The Absorb everolimus-eluting BVS is a fully bioresorbable scaffold. It consists of a poly(l-lactide) backbone coated with a poly(d,l-lactide) coating in a 1:1 ratio with everolimus [6]. The scaffold has a corrugated ring design, and is mounted on a Xience delivery catheter. The scaffold is radio-transparent, and two platinum markers are placed close to the proximal and distal edges of the device to allow its proper positioning [7]. The resorption process is mainly by hydrolysis; thus minimal or no inflammation can be observed [6, 8]. The final products of this process are carbon dioxide and water. The scaffold is covered with everolimus, a potent antiproliferative drug, which is released at the same rate and amount as the metallic Xience V (Abbott Vascular, Santa Clara, CA, USA) [7]. The Absorb BVS is a thick-strut scaffold, with an average strut thickness of 157 μm [7].
The resorption of scaffold from the coronary artery reestablishes physiologic vasomotion, restores vessel original geometry, and preserves laminar blood flow [7]. This in turn may limit the development of neoatherosclerosis. In contrast to the use of metallic stents, where late luminal loss deteriorates the early result of percutaneous coronary intervention, after deployment of the BVS, a late luminal gain has been observed. The absence of permanent foreign material potentially reduces the risk of late stent thrombosis, myocardial infarction, and sudden death [7]. Anatomically, the use of a BVS for lesions located in the midportion of the LAD artery, obtuse marginal branch or distal portion of the right coronary artery will potentially allow future bypass grafting [7]. Thus younger patients with long and diffuse lesions, such as those after cardiac transplant, would be suitable candidates.
Unfortunately, the use of a BVS in CAV has not been well studied as patients after cardiac transplant have been excluded from these trials. There was a case of successful BVS implantation under intravascular ultrasound guidance in a patient with CAV [9]. A center in Hungary is also assessing outcomes of CAV percutaneous coronary intervention with BVS under OCT guidance [10]. In addition, a prospective multicenter pilot study evaluating the use of everolimus-eluting BVS in the treatment of CAV is currently under way [11]. This study will likely provide further insight into the safety and efficacy of BVS use in CAV.
To date, this is one of few case reports outlining the use of BVS in CAV under OCT guidance. Recently, however, significant safety concerns have been raised, with BVS being associated with a higher incidence of device thrombosis compared with metallic stents [12]. As such, in Australia, BVS use will be available only through clinical trials and registries.

