Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has high invasiveness and has emerged as a global pandemic and public health crisis, and the numbers of infections and deaths are rising [1, 2]. Recent research found that cardiac injury is associated with a high risk of in-hospital death among patients with COVID-19 in Wuhan, China [3–5]. Some research shows that elevated levels of inflammatory biomarkers are linked to the severity of COVID-19 [6–8]. However, there are limited data on the relationship between cardiac injury and inflammatory biomarkers in patients with severe COVID-19. This study aims to examine the potential relationship between cardiac injury and inflammatory biomarkers by retrospectively analyzing data from patients with severe COVID-19 in a hospital in Wuhan.
Methods
Study Participants
The single-center, retrospective, observational study was performed at Tongji Hospital, Wuhan, which was supported by the medical team of Beijing Hospital. We collected data from 100 patients from February 8 to March 31, 2020. COVID-19 was diagnosed in the patients according to “Diagnosis and Treatment of Corona virus Disease-19 (7th trial edition).” [9]. All patients were classified as having severe COVID-19. Diagnosis of severe COVID-19 required at least one of the following criteria to be met: (1) dyspnea, respiratory frequency of 30 or greater minutes; (2) blood oxygen saturation of 93% or less at rest; (3) PaO2/FiO2 ratio of 300 or less. Diagnosis of critical COVID-19 required at least one of the following criteria to be met: (1) respiratory failure with mechanical ventilation; (2) septic shock; (3) transfer to the intensive care unit because of multiple-organ failure [9]. Real-time reverse transcription PCR was used to confirm SARS-CoV-2 infection in all patients. Nine patients were excluded because of the lack of cardiac biomarkers, including the values of high-sensitivity troponin I (hsTnI) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP). All patients were discharged up to March 31, 2020, except for the patients who died.
The 91 patients included in the analysis were divided into a cardiac injury group (n=22) and a non-cardiac-injury group (n=69) according to the serum levels of hsTnI and NT-proBNP. Cardiac injury was defined as plasma hsTnI level greater than 34.2 pg/mL and/or NT-proBNP level greater than 450 pg/mL for patients younger than 50 years, greater than 900 pg/mL for patients aged between 50 and 75 years, and greater than 1800 pg/mL for patients older than 75 years [10].
Data Collection
We collected and analyzed the demographic characteristics (age and sex), clinical data (symptoms, comorbidities, vital signs on admission, laboratory examination results, treatment measures, time, and outcomes) from patients’ medical records. Laboratory examinations were conducted within 24 hours after admission, including a complete blood count, levels of inflammatory biomarkers, coagulation profile, renal and liver function, hsTnI level, and NT-proBNP level. Time included hospitalization and time from onset to admission. The inflammatory biomarkers included interleukin-6 (IL-6), IL-1β, IL-2 receptor (IL-2R), IL-8, IL-10, procalcitonin, ferritin, tumor necrosis factor, and high-sensitivity C-reactive protein (hsCRP). Measurements were repeated according to a change in illness while patients were in the hospital. The longest observed duration in survivors was 48 days.
Statistical Analysis
Continuous variables are expressed as the mean±standard deviation or the median and the interquartile range, and categorical variables are expressed as the number and the percentage. The means of continuous variables were compared by use of the independent group t test or the Mann-Whitney U test, as appropriate. The Pearson correlation coefficient and Spearman rank correlation were used for linear correlation analysis. Proportions for categorical variables were compared by the χ² test or Fisher’s exact test. Logistic regression analysis was used to further determine the predictors of cardiac injury. Statistical analyses were performed and figures were constructed with use of IBM SPSS Statistics (version 25) for all analyses. A two-sided α of less than 0.05 was considered statistically significant.
Result
A total of 91 patients between February 8 and March 31, 2020 were included in the analysis. Twenty-two patients (24.18%) had cardiac injury. The mean age of all patients was 61 years±14 years, ranging from 18 to 91 years. The most frequent initial symptom was fever (62 patients [68.1%]) (Table 1). In all patients, hypertension (37 patients [40.7%]) was the most frequent comorbidity. Patients with cardiac injury stayed longer in the hospital than those without cardiac injury. By April 10, 2020, four patients (4.4%) had died, all in the non-cardiac-injury group.
Demographic and Clinical Characteristics of Patients with Coronavirus Disease 2019 (COVID-19).
| Characteristic | All patients (n=91) | Patients with cardiac injury (n=22) | Patients without cardiac injury (n=69) | P |
|---|---|---|---|---|
| Age, years, mean (SD) | 61 (14) | 65 (17) | 59 (13) | 0.155 |
| Male | 52 (57.1%) | 17 (77.3%) | 35 (50.7%) | 0.052 |
| Initial symptoms | ||||
| Fever | 62 (68.1%) | 11 (50%) | 51 (73.9%) | 0.067 |
| Cough | 56 (61.5%) | 12 (54.5%) | 44 (63.8%) | 0.601 |
| Dyspnea | 5 (5.5%) | 4 (18.2%) | 1 (1.4%) | 0.014 |
| Headache | 4 (4.4%) | 1 (4.5%) | 3 (4.3%) | 1.000 |
| Sore throat | 3 (3.3%) | 1 (4.5%) | 2 (2.9%) | 1.000 |
| Muscle ache | 4 (4.4%) | 1 (4.5%) | 3 (4.3%) | 1.000 |
| Chest distress | 13 (14.3%) | 8 (36.4%) | 4 (5.8%) | 0.665 |
| Chest pain | 2 (2.2%) | 1 (4.5%) | 1 (1.4%) | 0.978 |
| Fatigue | 11 (12.1%) | 5 (22.7%) | 6 (8.7%) | 0.167 |
| Nausea | 2 (2.2%) | 2 (9.1%) | 0 (0%) | 0.09 |
| Comorbidities | ||||
| Hypertension | 37 (40.7%) | 15 (68.2%) | 22 (31.9%) | 0.006 |
| CHD | 12 (13.2%) | 7 (31.8%) | 5 (7.2%) | 0.007 |
| Diabetes | 20 (22%) | 8 (36.4%) | 12 (17.4%) | 0.078 |
| Cerebrovascular disease | 4 (4.4%) | 0 (0%) | 4 (5.8%) | 0.577 |
| Chronic lung disease* | 12 (13.2%) | 2 (9.1%) | 10 (14.5%) | 0.723 |
| Endocrine and metabolic diseases† | 5 (5.7%) | 3 (15.8%) | 2 (2.9%) | 0.112 |
| Cancer | 12 (13.2%) | 4 (18.2%) | 8 (11.6%) | 0.474 |
| Vital signs on admission | ||||
| SBP, mmHg, mean (SD) | 136 (17) | 140 (17) | 134 (18) | 0.223 |
| DBP, mmHg, mean (SD) | 83 (12) | 85 (11) | 82 (12) | 0.421 |
| HR, bpm, median (IQR) | 93 (78–102) | 88 (75–101) | 94 (80–103) | 0.151 |
| Spo 2 ‡, %, median (IQR) | 97 (94–98) | 96 (93–97) | 97 (95–99) | 0.022 |
| Breathing rate, breaths per minute, median (IQR) | 20 (19–22) | 20 (19–24) | 20 (19–21) | 0.196 |
| Therapy | ||||
| Antiviral | 83 (91.2%) | 20 (90.9%) | 63 (91.3%) | 1.000 |
| Antibiotic | 33 (36.3%) | 7 (31.8%) | 25 (37.7%) | 0.808 |
| Traditional Chinese medicine | 55 (60.4%) | 14 (63.6%) | 41 (59.4%) | 0.919 |
| Time | ||||
| From onset to admission, days, median (IQR) | 14 (7–28) | 10 (4–20) | 14 (10–28) | 0.078 |
| Hospitalization, days, mean (SD) | 28 (15) | 35 (15) | 25 (15) | 0.013 |
| Death | 4 (4.4%) | 0 (0%) | 4 (5.8%) | 0.569 |
Data are shown as the mean and the standard deviation (SD) in parentheses, the median and the interquartile range (IQR) in parentheses, or the number and the percentage in parentheses.
CHD, coronary heart disease; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure; Spo2, percutaneous oxygen saturation.
*Including chronic bronchitis, bronchiectasis, emphysema, tuberculosis, and chronic obstructive pulmonary disease.
†Including hyperthyroidism, hypothyroidism, and hyperlipidemia.
‡Oxygen saturation was measured on admission after the patient had received oxygen therapy.
Patients in the cardiac injury group had significantly higher levels of hsTnI and NT-proBNP, as expected (Table 2). The white blood cell count, neutrophil count, prothrombin time, thrombin time, D-dimer level, and creatinine level were higher in the patients with cardiac injury than in the patients without cardiac injury (Table 2).
Laboratory Findings for Different Groups of Patients.
| Characteristic | With cardiac injury (n=22) | Without cardiac injury (n=69) | P |
|---|---|---|---|
| hsTnI, pg/mL | 14.60 (3.88–64.68) | 2.70 (1.90–6.70) | <0.001 |
| NT-proBNP, pg/mL | 773.50 (516.5–1546.5) | 76.00 (31.25–189.25) | <0.001 |
| Complete blood cell count | |||
| White blood cell count, ×109/L | 8.64 (4.58–10.44) | 5.79 (4.12–7.40) | 0.041 |
| Neutrophil count, ×109/L | 7.40 (3.05–8.63) | 3.72 (2.33–5.28) | 0.005 |
| Lymphocyte count, ×109/L | 0.91 (0.74–1.31) | 1.23 (0.72–1.66) | 0.478 |
| Platelet count, ×109/L | 185 (149–287) | 231 (174–302) | 0.126 |
| Hemoglobin, g/L | 121 (102–139) | 122 (108–132) | 0.771 |
| Coagulation profile | |||
| PT, s | 14.3 (13.5–15.3) | 13.9 (13.4–14.1) | 0.049 |
| APTT, s | 39.3 (37.8–45.1) | 39.0 (35.7–41.7) | 0.325 |
| TT, s | 16.7 (15.5–18.9) | 15.7 (15.2–16.7) | 0.035 |
| Fibrinogen, g/L | 4.85 (3.82–5.97) | 4.9 3 (4.03–5.89) | 0.725 |
| D-dimer, mg/L | 2.31 (1.03–9.40) | 0.99 (0.40–2.66) | 0.011 |
| Renal and liver function | |||
| ALT, U/L | 28 (12–43) | 19 (12–40) | 0.291 |
| Creatinine, μmol/L | 77 (62–104) | 63 (54–82) | 0.047 |
| eGFR, mL/(min 1.73 m2) | 86.2 (57.4–100.8) | 96.1 (82.5–104.2) | 0.054 |
Data are shown as the median and the interquartile range in parentheses.
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; eGFR, estimated glomerular filtration rate; hsTnI, high-sensitivity troponin I; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PT, prothrombin time; TT, thrombin time.
Table 3 shows that the peak values of inflammatory biomarkers in the cardiac injury group were generally higher than those in the group without cardiac injury. Although the IL-8 level was higher in patients without cardiac injury, the levels in both groups were within the normal range (<62 pg/mL). Figures 1 and 2 shows that there are positive correlations between the levels of hsTnI and NT-proBNP and the levels of inflammatory biomarkers.
Peak Levels of Inflammatory Biomarkers for Different Groups of Patients in the Hospital.
| Inflammatory biomarker | With cardiac injury (n=22) | Without cardiac injury (n=69) | P |
|---|---|---|---|
| hsCRP, pg/mL | 68.70 (11.33–110.60) | 11.50 (2.75–48.85) | 0.013 |
| Procalcitonin, pg/mL | 0.14 (0.07–0.44) | 0.07 (0.03–0.12) | 0.008 |
| TNF, pg/mL | 10.80 (8.65–16.45) | 9.40 (7.65–12.65) | 0.110 |
| Ferritin, ng/mL | 889.15 (387.78–1581.95) | 515.40 (303.60–1551.70) | 0.220 |
| Interleukin-1β, pg/mL | 5.00 (5.00–5.80) | 5.00 (5.00–5.28) | 0.303 |
| Interleukin-2 receptor, U/mL | 954.00 (723.50–1451.5) | 518.00 (339.50–815.00) | <0.001 |
| Interleukin-6, pg/mL | 38.09 (16.09–65.88) | 7.18 (2.93–25.40) | 0.001 |
| Interleukin-8, pg/mL | 26.30 (16.30–66.40) | 16.50 (8.05–34.95) | 0.03 |
| Interleukin-10, pg/mL | 5.00 (5.00–12.15) | 5.00 (5.00–6.85) | 0.235 |
Data are shown as the median and the interquartile range in parentheses. The normal values are as follows: high-sensitivity C-reactive protein (hsCRP), less than 30 pg/mL; procalcitonin, 0.02–0.05 pg/mL; tumor necrosis factor (TNF), less than 8.1 pg/mL; ferritin, 15–150 ng/mL; interleukin-1β, less than 5.0 pg/mL; interleukin-2 receptor, 223–710 U/mL; interleukin-6, less than 7.0 pg/mL; interleukin-8, less than 62 pg/mL; interleukin-10, less than 9.1 pg/mL.
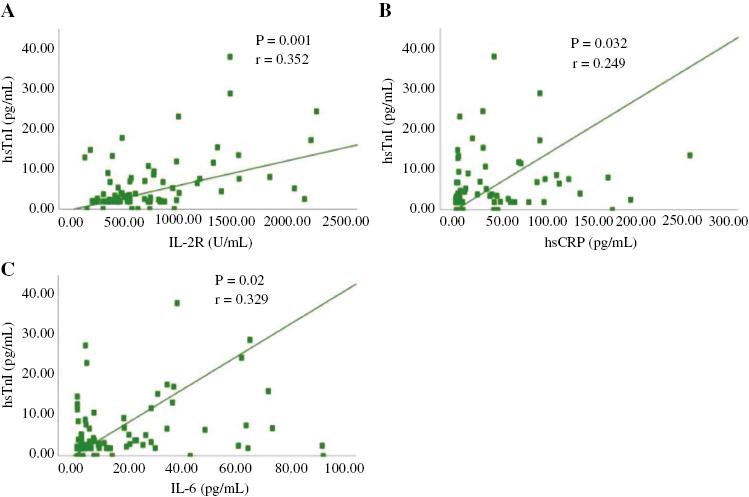
Correlation between the Levels of High-Sensitivity Troponin I (hsTnI) and Inflammatory Biomarkers.
(A) There are positive correlations between the levels of hsTnI and interleukin-2 receptor (IL-2R) (P=0.001, r=0.352); (B) There are positive correlations between the levels of hsTnI and high-sensitivity C-reactive protein (hsCRP) (P=0.032, r=0.249); (C) There are positive correlations between the levels of hsTnI and interleukin-6 (IL-6) (P=0.02, r=0.556).
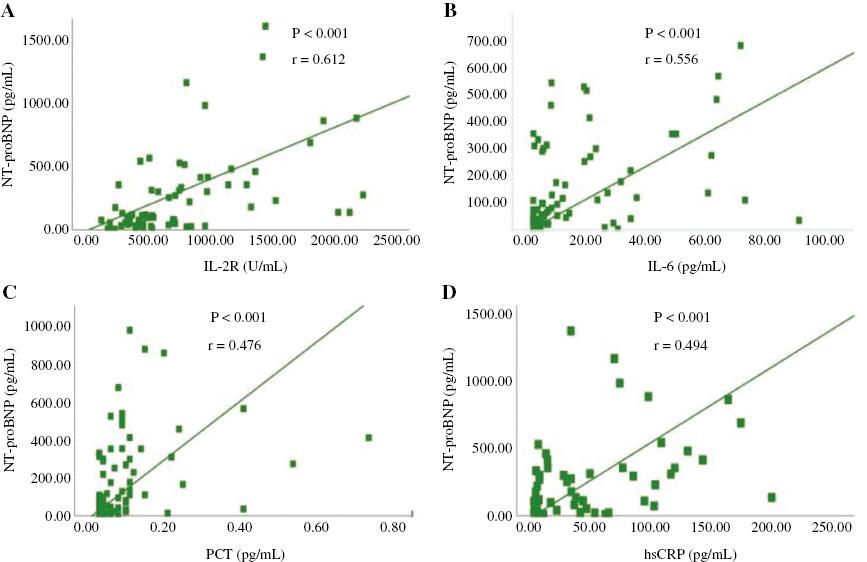
Correlation between the Levels of N-terminal Prohormone of Brain Natriuretic Peptide (NT-proBNP) and Inflammatory Biomarkers.
(A) There are positive correlations between the levels of NT-proBNP and interleukin-2 receptor (IL-2R) (P<0.001, r=0.612); (B) There are positive correlations between the levels of NT-proBNP and interleukin-6 (IL-6) (P<0.001, r=0.556); (C) There are positive correlations between the levels of NT-proBNP and procalcitonin (PCT) (P<0.001, r=0.476); (D) There are positive correlations between the levels of NT-proBNP and high-sensitivity C-reactive protein (hsCRP) (P<0.001, r=0.494).
Logistic regression analysis was used to assess the effect of age, male sex, comorbidities, IL-2R, and creatinine on cardiac injury in patients with severe COVID-19. Comorbidities included hypertension, coronary heart disease, and diabetes. In univariable logistic regression analysis, male sex, comorbidities, IL-2R and creatinine were risk factors for cardiac injury (Table 4). Multivariable regression analysis showed IL-2R (odds ratio 1.001, 95% confidence interval 1.000–1.002, P=0.023) and comorbidities (odds ratio 4.909, 95% confidence 1.231–19.579, P=0.024) are independent risk factors for cardiac injury in patients with severe COVID-19 (Table 4).
Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Cardiac Injury.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Male sex | 0.303 | 0.100–0.912 | 0.034 | 0.320 | 0.081–1.265 | 0.104 |
| Age | 1.025 | 0.990–1.061 | 0.160 | 0.987 | 0.940–1.036 | 0.596 |
| IL-2R | 1.001 | 1.000–1.002 | 0.007 | 1.001 | 1.000–1.002 | 0.023 |
| Creatinine | 1.018 | 1.002–1.034 | 0.025 | 1.007 | 0.989–1.025 | 0.475 |
| Comorbidities* | 4.148 | 1.44–11.92 | 0.008 | 4.909 | 1.231–19.579 | 0.024 |
CHD, coronary heart disease; CI, confidential interval; IL-2R, interleukin-2 receptor, OR, odds ratio.
*Including hypertension, coronary heart disease, and diabetes.
Discussion
This retrospective cohort study elicited three main findings. First, the occurrence of cardiac injury is not uncommon, and the incidence reaches 24.18% in patients with severe COVID-19. Second, elevated levels of inflammatory biomarkers and cardiovascular disease are more prevalent in the cardiac injury group. Finally, IL-2R and preexisting cardiovascular disease are independent risk factors for cardiac injury in patients with severe COVID-19.
Several retrospective studies exploring the relationship between cardiac injury and mortality in COVID-19 patients have been published. Shi et al. [3] and Guo et al. [11] both reported COVID-19 patients with cardiac injury have higher levels of C-reactive protein and procalcitonin. Consistently, our study also found that patients with severe COVID-19 with cardiac injury have evidence of severer systemic inflammation, including higher levels of IL-6, IL-2R, hsCRP, and procalcitonin. Our study participants were mainly patients with severe COVID-19. Moreover, our research provides information not reported by previous research, and first demonstrates that IL-2R and underlying cardiovascular disease are independently associated with increased risk of cardiac injury in patients with severe COVID-19.
Huang et al. [6] found that COVID-19 patients admitted to the intensive care unit (ICU) had higher plasma levels of cytokines, including IL-2, IL-7, IL-10, and tumor necrosis factor than those non-ICU patients. This indicates that critical COVID-19 patients may experience the cytokine storm, an uncontrolled and dysfunctional immune response [12, 13]. In the present study, the levels of inflammatory biomarkers had positive correlations with the levels of hsCRP and NT-proBNP in patients with severe COVID-19, suggesting that a cytokine storm may contribute to myocardial injury and left ventricular impairment. Patients with cardiac injury had higher levels of inflammatory biomarkers, including white blood cell count, neutrophil count, hsCRP level, and procalcitonin level. So we speculate that inflammation plays an important role in cardiac injury. Inflammation may cause endothelial cell dysfunction and increase the procoagulant activity of blood, which can lead to the rupture of coronary plaque [14–17].
Among all the cytokines studied, the effect of IL-2R on cardiac injury is particularly prominent. IL-2, a T-cell growth factor, and IL-2R have been found in plaque [18, 19]. Previous research found that IL-2R regulates lymphocyte activation, plays an important role in atherosclerosis, and positively relates to the occurrence of cardiovascular disease [20–22]. IL-2 promotes regulatory T cells and may have an atheroprotective role [23]. So we presume that because of SARS-CoV-2 invasion and lymphopenia [24], the interaction of excessively secreted IL-2 and IL-2R promotes the development of atherosclerosis and advanced cardiac injury.
By April 10, 2020, 95.6% of patients in our study had been discharged from hospital, and mortality was 4%. Although we did not find statistical differences in mortality regarding patients with severe COVID-19 with or without cardiac injury on admission, patients with cardiac injury stayed longer in the hospital than patients without cardiac injury. Long-term observation is needed to confirm the impact of elevated levels of inflammation biomarkers on the prognosis of patients with severe COVID-19 with cardiac injury.
Limitations
Our research has several limitations. Firstly, it was a small sample, single-center, and retrospective study, and selection bias may not be avoided in this study. At the same time, a small sample size might lead to overfitting. Secondly, some results of cardiac examinations, such as electrocardiogram and cardiac ultrasonography, are incomplete in our study, resulting in our being unable to comprehensively describe the cardiac function. Thirdly, because of quick outbreaks and urgent treatment needs, changes in the levels of inflammatory biomarkers were not detected regularly. Data from larger populations and multiple centers are warranted to confirm the dynamic change of the levels of inflammatory biomarkers.

