Introduction
Heart failure remains a leading health issue in the aged population worldwide. Acute decompensated heart failure (ADHF), the rapid onset of or change in the symptoms and signs of heart failure, is the most common cause of heart failure hospitalization and cardiac death [1, 2]. The hospitalization-related costs of ADHF account for 75% of the total cost of heart failure care [3]. Despite therapeutic progress in the care of patients with chronic heart failure, the rates of early death and rehospitalization in ADHF patients have not reduced over the past several decades [4, 5]. Hence, improvements in the care of patients with ADHF would have an extensive influence on health care delivery.
Although not “evidence based” in the same manner as treatments for chronic heart failure, the key therapy for ADHF consists predominantly of oxygen, diuretics, and vasodilators. Diuretics play an indispensable role in decongestion and are thus recommended (class I, level of evidence B or C) for this reason in patients with ADHF manifesting the symptoms and signs of volume overload [3, 6]. Although intravenous diuretics are the first-line medication for the treatment of ADHF and are prescribed for approximately 90% of ADHF inpatients [7], the efficacy of intravenous loop diuretics in relieving volume retention is limited by neurohormonal activation, deterioration of renal function, and diuretic resistance [8, 9]. Given these limitations, a growing number of clinicians are interested in alternative approaches to manage congestion in patients with ADHF effectively and safely. Extracorporeal ultrafiltration is a potentially promising treatment strategy to manage volume overload in ADHF [10]. This therapeutic option has several potential advantages, including better control of fluid removal in terms of rate and volume, greater sodium removal, and less neurohormonal activation [11]. Currently, ultrafiltration is recommended as a reasonable approach in congestive patients who are unresponsive to medication treatment in accordance with American College of Cardiology Foundation/American Heart Association guidelines (class IIa, level of evidence B, or class IIb, level of evidence B) [6, 12]. However, the efficacy and renal tolerability of ultrafiltration remain controversial in patients with ADHF compared with standard medical therapy. Previous studies indicated that ultrafiltration was efficient and safe for the treatment of ADHF with respect to its superiority with regard to volume management and preservation of renal function [13, 14]. Nevertheless, a recent study found that ultrafiltration for ADHF therapy was associated with increases in serum creatinine levels and adverse events and did not have favorable effects on weight loss or total fluid output compared with intravenous diuretics [15]. This meta-analysis of randomized controlled trials (RCTs) was consequently performed to evaluate the efficacy and renal tolerability of ultrafiltration in ADHF.
Methods
Search Strategy
An electronic search of PubMed, EMBASE, and the Cochrane database of controlled trials (up to March 2021) was performed to identify eligible RCTs evaluating the ultrafiltration strategy in ADHF patients. We used the following keywords and corresponding MeSH terms without language limitation: “ultrafiltration,” “heart failure,” “cardiac failure,” “cardiac dysfunction,” “cardiac insufficiency,” “decompensated heart failure,” “ventricular dysfunction,” and “random*.” Additionally, reference lists of eligible studies and review articles were also searched.
Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: (1) the study participants were patients with ADHF; (2) the studies were prospective, randomized, controlled clinical trials; (3) the intervention consisted of ultrafiltration therapy; and (4) weight loss, fluid removal, change in serum creatinine level, change in serum N-terminal prohormone of brain natriuretic peptide (NT-proBNP) level, change in serum sodium level, length of hospital stay, all-cause rehospitalization, and all-cause mortality were reported as clinical outcomes. The exclusion criteria were as follows: ultrafiltration was performed as an adjunctive therapy, repeated data were included, or no outcomes of interest were reported.
Quality Assessment and Data Extraction
The methodological quality of each study was assessed with the use of the Cochrane Collaboration’s risk of bias assessment tool. Two investigators independently reviewed all potentially eligible studies and extracted data on studies and patient characteristics. Any disagreement or uncertainty was resolved by discussion and consensus.
Data Synthesis and Analysis
The primary end points included (1) change in the serum creatinine level, (2) all-cause mortality, (3) all-cause rehospitalization, and (4) length of hospital stay. The secondary end points included (1) weight loss, (2) fluid removal, (3) change in serum NT-proBNP level, and (4) change in serum sodium level. The risk ratio (RR) was used to analyze dichotomous variables with the 95% confidence interval (CI), whereas the weighted mean difference (WMD) or the standardized mean difference (SMD) with the 95% CI was used to analyze continuous data. Fixed-effects models were used for pooled analyses, whereas in the case of significant heterogeneity, random-effects models were applied. The χ 2 test and the I 2 statistic were used to test and quantify statistical heterogeneity, respectively. Studies with an I 2 value of 25–50%, 50–75%, or more than 75% are considered to have a low, moderate, or high degree of heterogeneity, respectively [16]. An I 2 value greater than 50% indicates significant heterogeneity. Sensitivity analyses were performed to evaluate the stability of outcomes and to explore the influence of study design and patient characteristics on the effect size. Additionally, a subgroup analysis was conducted on the basis of age, follow-up duration, and left ventricular ejection fraction. RevMan 5.4 (Cochrane Collaboration, Copenhagen, Denmark) was used to perform statistical analyses [17]. Statistical significance was set at the two-tailed P<0.05 level for any test or model.
Assessment of Outcome Quality
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to evaluate the evidence level and was performed according to study design, risk of bias, indirectness, inconsistency, publication bias, and imprecision. We performed the study in accordance with the reporting quality for meta-analyses (PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-analyses] Statement). The evaluation was performed with the use of GRADEpro 3.6 (Cochrane Collaboration).
Results
Results of Search and Description of Studies
The flowchart used to select studies for this meta-analysis is illustrated in Figure 1. We identified 1201 records, from which 223 RCTs were excluded for duplication and 887 studies were excluded on the basis of the title and the abstract. For a more detailed assessment, the remaining 91 articles with full text along with ten additional articles identified from the reference and citation reviews were reviewed; 82 of these studies were excluded after the full article review because 39 articles did not report relevant outcomes, 29 studies were nonrandomized studies, six studies had no placebos, six studies were ongoing, and two articles were duplicate publications from the same trial. Finally, 19 RCTs were eligible and analyzed in our meta-analysis [10, 13–15, 18–32].
The characteristics of the studies included in the meta-analysis are shown in Table 1. Of the 1281 participants in total, 620 patients were assigned to the ultrafiltration group and 661 patients were assigned to the control group. Figures 2 and 3 show the risk of bias in the included RCTs. Only seven of the 19 RCTs described the randomization method in detail. Allocation concealment was reported in four included studies. None of the included studies described blinding. However, the risk of bias in incomplete outcome data was low in most of the included studies. Other potential sources of bias may be due to a relatively small sample size and trials conducted by the same research organization.
Main Characteristics of the 19 Randomized Controlled Trials Included in the Meta-Analysis.
| Study | N (I/C) | Age (mean, years) (I/C) | Male (%) (I/C) | LVEF (%, mean) (I/C) | NYHA FC | Intervention group | Control group | Follow-up (mean) |
|---|---|---|---|---|---|---|---|---|
| Agostoni et al. [18] | 18/18 | 57.8/59.1 | 88.9/77.8 | 23.8/24.1 | II–III | UF (rate 600 mL/h, duration 188±8 ms) | No UF | 180 days |
| Agostoni et al. [26] | 8/8 | 58/62 | 87.5/87.5 | NA | II–III | UF (rate 500 mL/h) | Intravenous furosemide | 90 days |
| Agostoni et al. [20] | 21/21 | 61/61 | NA | 27/25 | NA | UF (rate 600 mL/h) | No UF | 90 days |
| Bart et al. [21] | 20/20 | 67.5/69.5 | 70/70 | 69%/78%* | III–IV | UF (rate ≤500 mL/h, duration 8 h) plus usual care | Standard HF therapies | 30 days |
| Bart et al. [15] | 94/94 | 69/66 | 78/72 | 30/35 | NA | UF (rate 200 mL/h, median duration 40 h) | Intravenous diuretic and possible use of intravenous vasodilators and inotropic agents | 60 days |
| Chung et al. [24] | 8/8 | 69/74 | 87.5/100 | 22/26 | III–IV | UF | Intravenous furosemide and intravenous vasodilators or inotropes | 90 days |
| Costanzo et al. [10] | 100/100 | 62/63 | 70/68 | 71%/70%* | II–IV | UF (rate up to 500 mL/h) | Intravenous diuretic | 90 days |
| Cosentino et al. [27] | 5/7 | 78.8/72.8 | NA | 39/35 | NA | UF | Conventional management | 1 year |
| Giglioli et al. [14] | 15/15 | 72.4/65.8 | 87/87 | 34/30 | III–IV | UF (rate 100–300 mL/h) | Intravenous furosemide | 36 h after treatment |
| Hanna et al. [23] | 19/17 | 60/59 | 84.2/76 | 19/18 | III–IV | UF (rate 400 mL/h for 6 h, then 200 mL/h) and possible use of intravenous vasoactive medications | Intravenous loop diuretics and possible use of intravenous vasoactive medications | 90 days |
| Marenzi et al. [25] | 27/29 | 75/73 | 81/83 | 32/32 | III–IV | UF (rate 100–500 mL/h) | Standard medical therapy | 1 year |
| Pepi et al. [19] | 12/12 | 57/56 | NA | 23.8/24.2 | NA | UF (rate 600 mL/h) | No UF | 90 days |
| Rogers et al. [22] Costanzo et al. [29] | 9/10 110/114 | 64/54 67/67 | 78/60 69.1/73.0 | <40 36.3/36.6 | II–III NA | UF (rate 10–40 mL/min) UF (rate 50–300 mL/h) | Intravenous furosemide Furosemide-equivalent intravenous loop diuretics | 40 h 90 days |
| Tabak’ian et al. [32] | 19/21 | NA | NA | NA | II–IV | Slow continuous UF | Intravenous furosemide | NA |
| Badawy and Fahmy [13] | 20/20 | 64/62 | 70/60 | 75%/70%* | III–IV | UF (rate: 35 mL/kg h) | Intravenous furosemide | 30 days |
| Seker et al. [28] | 10/20 | 66.5 | 60 | 31 | NA | UF | Intravenous furosemide | 30/90 days |
| Shen et al. [31] | 65/67 | 58/57 | 70.1/67.7 | NA | NA | UF (rate: 300–500 mL/h) | Intravenous furosemide and other standard HF therapies | 12 h |
| Hu et al. [30] | 40/60 | 70/73 | 55/55 | NA | NA | UF (rate: 200–300 mL/h) | Torasemide plus tolvaptan | 7 days |
C, control group; HF, heart failure; I, intervention group; LVEF, left ventricular ejection fraction; NA, not available; NYHA FC, New York Heart Association functional class; UF, ultrafiltration.
*Proportion of patients with LVEF less than 40%.
Outcomes of Renal Tolerability and Prognosis
The aggregated results of ten studies [10, 13–15, 22–25, 29, 30] suggested that ultrafiltration treatment significantly increased serum creatinine levels compared with the control treatments (SMD 0.15, 95% CI 0.00–0.30, P=0.04; Figure 4), without statistical heterogeneity across the trials (I 2=33%). Sensitivity analyses suggested that this adverse effect was concealed when the study by Bart et al. [15] was omitted. The instability may potentially be due to the study by Bart et al., whose participants were patients with worsened renal function.
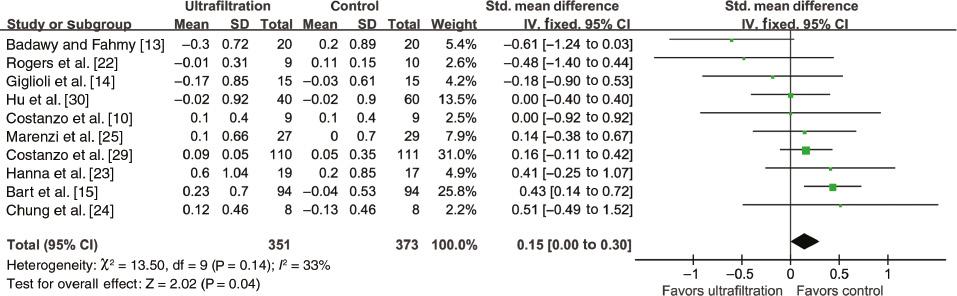
Forest Plot for Serum Creatinine Level Change.
CI, confidence intervals; df, degrees of freedom; IV, inverse variance; SD, standard deviation; Std., standardized.
The meta-analysis of 16 RCTs [10, 14, 15, 18–27, 29–31] showed no significant difference between the compared groups for all-cause mortality (RR 1.00, 95% CI 0.72–1.40, P=1.00, I 2=0%; Figure 5). Sensitivity analyses confirmed the results regarding all-cause mortality. In addition, the random-effects model yielded results similar to those of the fixed-effects model. Additionally, the results indicated all-cause rehospitalization (RR 0.84, 95% CI 0.56–1.26, P=0.39; Figure 6) and length of hospital stay (WMD 0.16, 95% CI −0.65 to 0.98, P=0.7; Figure 7) were not significantly different between groups, with significant heterogeneity (I 2=54% and 76%, respectively). In the analysis of length of hospital stay, exclusion of the study by Costanzo et al. [29] reduced heterogeneity from 76% to 0%, while the results were still not significant (WMD −0.17, 95% CI −0.72 to 0.37, P=0.54) However, sensitivity analyses suggested a trend toward decreased rehospitalization in the ultrafiltration group when the study by Bart et al. [15] was omitted.
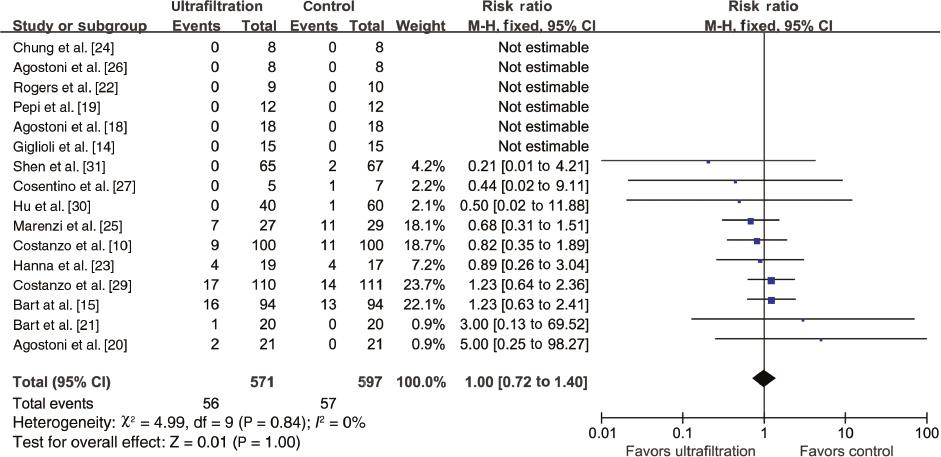
Forest Plot for All-Cause Mortality.
CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; SD, standard deviation.
Outcomes of Fluid Management and Other Laboratory Indexes
The pooled results of 1054 patients from 12 RCTs [10, 13–15, 22–25, 28–31] indicated that ultrafiltration was superior to the control treatments for weight loss (WMD 1.24, 95% CI 0.38–2.09, P=0.004; Figure 8), with significant heterogeneity (I 2=61%). Sensitivity analyses confirmed the results regarding weight loss. In addition, the fixed-effects model yielded results similar to those of the random-effects model. Likewise, a beneficial effect was detected for fluid removal in the ultrafiltration group (WMD 1.55, 95% CI 0.51–2.59, P=0.003; Figure 9), and sensitivity analyses confirmed the results.
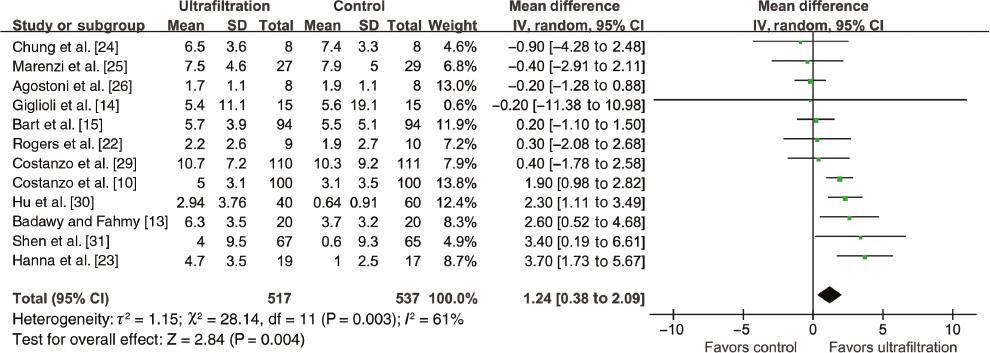
Forest Plot for Weight Loss.
CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
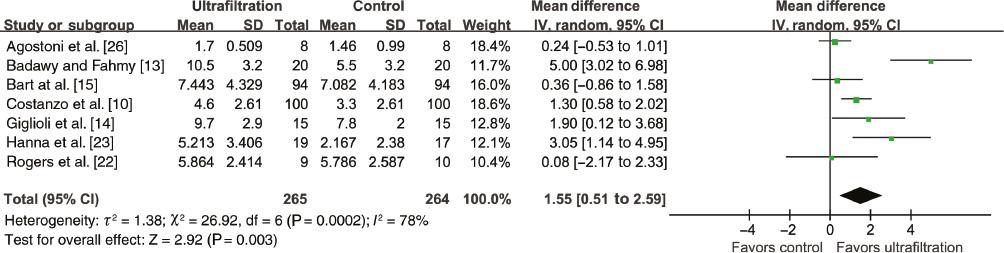
Forest Plot for Fluid Removal.
CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
No significant difference was reported between the compared groups in the change in serum NT-proBNP level (WMD −0.52, 95% CI −1.96 to 0.91, P=0.48, I 2=0%; Figure 10). However, the results showed that ultrafiltration was associated with a significant change in serum sodium level compared with the control treatments (WMD −1.56, 95% CI −2.33 to −0.80, P<0.0001, I 2=32%; Figure 11). This beneficial effect was concealed when the study by Bart et al. [15] was omitted. The study by Bart et al. enrolled patients with worsened renal function, who are likely to have diuretic resistance, with less removal of serum sodium.
Evidence of GRADE Profile
Table 2 shows the assessment of outcome quality and the reasons for grading. On the basis of the GRADE system, the evidence quality for the change in serum creatinine level and all-cause rehospitalization was low, whereas the evidence quality for all-cause mortality, weight loss, and fluid removal was moderate.
Summary of GRADE Evidence Profile.
|
Patient or population: patients with ADHF Settings: inpatients Intervention:ultrafiltration | |||||
|---|---|---|---|---|---|
| Outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants | Quality of the evidence (GRADE) | |
| Assumed risk (control) | Corresponding risk (ultrafiltration) | ||||
| Weight loss | The mean weight loss across control groups ranged from 0.6 to 10.3 kg | The mean weight loss in the intervention groups was 1.24 kg higher than that of the control group | 1054 (12 studies) | ⊕⊕⊕⊝ moderate | |
| Fluid removal | The mean fluid removal across control groups ranged from 1.4 to 7.8 l | The mean fluid removal in the intervention groups was 1.55 L higher than that of the control group | 529 (7 studies) | ⊕⊕⊕⊝ moderate | |
| Serum creatinine level change | The mean serum creatinine level change across control groups ranged from −0.13 to 0.2 mg/dL | The mean serum creatinine level change in the intervention groups was 0.15 standard deviations higher (0.00–0.30 higher) | 724 (10 studies) | ⊕⊕⊝⊝ low | |
| All-cause mortality | 95 per 1000 | 98 per 1000 (90–210) | RR 1.0 (0.72–1.4) | 1168 (16 studies) | ⊕⊕⊕⊝ moderate |
| All-cause rehospitalization | 332 per 1000 | 229 per 1000 (175–500) | RR 0.69 (0.49–0.99) | 396 (6 studies) | ⊕⊕⊝⊝ low |
ADHF, acute decompensated heart failure; CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; RR, risk ratio.
*The basis for the assumed risk (e.g., the median control group risk across studies) is provided below. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Subgroup Analysis
Subgroup analysis showed that patients younger than 65 years were more likely to benefit from ultrafiltration with respect to weight loss in the present meta-analysis. Moreover, renal function in patients aged 65 years or older and with left ventricular ejection fraction of 30% or greater was more likely to be worsened by ultrafiltration treatment (Table 3). Furthermore, patients with a long follow-up duration (12 months) may experience more benefit with respect to all-cause rehospitalization than patients with a short follow-up (less than 12 months); nevertheless, such superiority did not exist for all-cause mortality. Besides, the adverse renal effects of ultrafiltration were concealed in patients with less worsened renal function (serum creatine level of 1.7 mg/dL or lower).
Subgroup Analysis.
| Age | Baseline LVEF | Follow-up | Baseline serum creatine level | |||||
|---|---|---|---|---|---|---|---|---|
| ≥65 years | <65 years | ≥30% | <30% | 12 | 1 month to <12 months | >1.7 mg/dL | ≤1.7 mg/dL | |
| Weight loss | ||||||||
| Studies/patients | 6/611 | 6/443 | 4/495 | 2/52 | – | – | 5/309 | 6/729 |
| WMD (95% CI) | 0.67 (−0.48 to 1.83) | 1.77 (0.46–3.08) | 0.14 (−0.87 to 1.16) | 1.61 (−2.87 to 6.10) | – – | – – | 0.03 (−0.96 to 1.02) | 2.17 (1.56–2.78) |
| P | 0.25 | 0.008 | 0.78 | 0.48 | – | – | 0.96 | <0.00001 |
| Serum creatine level change | ||||||||
| Studies/patients | 6/564 | 3/95 | 4/448 | 2/52 | – | – | 3/274 | 7/450 |
| WMD (95% CI) | 0.10 (0.01–0.18) | −0.13 (−0.32 to 0.07) | 0.10 (0.01–0.18) | 0.30 (−0.06 to 0.67) | – – | – – | 0.21 (0.05–0.36) | 0.03 (−0.03 to 0.09) |
| P | 0.03 | 0.20 | 0.03 | 0.10 | – | – | 0.008 | 0.40 |
| All-cause mortality | ||||||||
| Studies/patients | 8/663 | 8/505 | 5/507 | 5/149 | 2/68 | 10/819 | 3/274 | 8/764 |
| RR (95% CI) | 1.06 (0.72–1.57) | 0.87 (0.46–1.64) | 1.05 (0.71–1.57) | 1.33 (0.45–3.93) | 0.66 (0.31–1.42) | 1.16 (0.79–1.69) | 0.98 (0.59–1.64) | 0.97 (0.62–1.53) |
| P | 0.77 | 0.66 | 0.80 | 0.61 | 0.28 | 0.46 | 0.95 | 0.91 |
| All-cause rehospitalization | ||||||||
| Studies/patients | 5/367 | 2/212 | 3/251 | 2/52 | 2/68 | 3/235 | 2/239 | 4/328 |
| RR (95% CI) | 0.87 (0.52–1.47) | 0.76 (0.37–1.59) | 0.74 (0.30–1.80) | 1.24 (0.63–2.42) | 0.45 (0.23–0.87) | 1.28 (0.95–1.70) | 1.04 (0.63–1.72) | 0.68 (0.41–1.14) |
| P | 0.61 | 0.47 | 0.50 | 0.53 | 0.02 | 0.10 | 0.88 | 0.14 |
CI, confidence interval; LVEF, left ventricular ejection fraction; RR, risk ratio; WMD, weighted mean difference.
Discussion
Heart failure remains a significant public health issue worldwide because of the high associated mortality and cost [33]. Fluid retention and congestion are responsible for 90% of heart failure rehospitalizations [7], and severer congestion leads to worse outcomes [34]. Ultrafiltration, a mechanical method of fluid removal, may be a promising approach for volume management in ADHF patients, with potential advantages of better control of fluid removal, greater sodium removal, and less activation of the neurohormonal system [35]. However, the efficacy and renal tolerability of ultrafiltration in ADHF remain a controversial issue. This meta-analysis shows that ultrafiltration in patients with ADHF is superior for weight loss, fluid removal, and serum sodium removal, although it has adverse renal effects and lacks significant effects on prognosis. The findings of this updated meta-analysis are different from those of a previous meta-analysis indicating that the use of ultrafiltration was superior to standard medical therapy with respect to the hypervolume management of weight loss and total fluid output without worsening or preserving renal function in terms of changes in serum creatinine levels [36, 37].
The renal tolerability of ultrafiltration for patients with ADHF remains controversial but is gaining wide interest. Bart et al. [15] indicated treatment with ultrafiltration was associated with a higher rate of deterioration in renal function in the CARRESS-HF trial. However, in the CUORE trial, no differences were found in renal function between the ultrafiltration group and the control group [25]. The findings of our meta-analysis reveal that ultrafiltration significantly increases the serum creatinine level compared with the control treatments. These conflicting findings regarding adverse renal effects between the present meta-analysis and previous meta-analyses and studies could be explained by the following possibilities. First, differences in the inclusion criteria for the study population, renal function at the baseline, indications for ultrafiltration treatment, and the regimen of both ultrafiltration and conventional therapy among trials could explain some of these contradictory results. Second, the adverse renal effect in the present meta-analysis seems to derive largely from the most weighed trial, the CARRESS-HF trial by Bart et al. [15], a multicenter study with patients with ADHF complicated by worsened renal function and persistent congestion. The significant increase in serum creatinine level in the ultrafiltration group may be due to the fixed ultrafiltration rate of 200 mL/h and lack of a customized volume target based on the patients’ clinical status. The rate and total volume of ultrafiltration in the trial were excessive for patients with advanced diseases and could lead to an imbalance between intravascular water removal and capillary refill, potentially increasing the incidence of worsening renal function [35, 38]. Despite sensitivity analyses suggesting that this adverse effect of ultrafiltration treatment was concealed when the study by Bart et al. [15] was omitted, there was still a trend of worsening renal function in the ultrafiltration group compared with controls in the pooled analyses of the other nine studies [10, 13–14, 22–25, 29, 30], without statistical significance (SMD 0.05, 95% CI −0.12 to 0.22, P=0.54). Besides, some clinicians question the use of the serum creatinine level to assess renal function, for it may not be as accurate as cystatin C level or glomerular filtration rate [39, 40]. Whereas some recommend increased serum creatinine level as a variable reflecting a significant change in blood volume, in patients with sustained abnormality of blood volume, which may worsen renal function, serum creatinine level can reflect renal function as accurately as cystatin C level or glomerular filtration rate does. On the whole, there is evidence to refute the view that ultrafiltration is less harmful to renal function in patients with ADHF than the best first-line drug therapy according to the results of the present meta-analysis and clinical trials. Moreover, further well-designed and well-executed studies with the use of more accurate and consistent measurements for renal function assessment are needed to demonstrate the impact of ultrafiltration versus the best first-line drug therapy on renal function and to decide on the proper indications for ultrafiltration, the ideal rate and duration of fluid removal, and the best time for discontinuing this treatment.
In ADHF patients, hypervolemia and congestion are major characteristics and contribute to heart failure progression and prognosis, and treatment guidelines recommend that treatment be aimed at decongestion and achieving euvolemia [6]. For initial therapy in patients with ADHF, there are some clinical studies and meta-analyses showing the beneficial effects of ultrafiltration with regard to fluid retention management in terms of weight loss and total fluid output compared with drug therapy [10, 23, 36, 37]. Our meta-analysis confirmed that ultrafiltration is associated with significant effects on weight loss and fluid removal. There was substantial heterogeneity across the RCTs involving weight loss and fluid removal comparison, which was reasonable given the huge differences in patient characteristics, detailed treatment strategies, and study designs.
The available data in the present meta-analysis demonstrate that ultrafiltration does not contribute to a reduction in serum NT-proBNP level, which not only represents a marker of both myocardial damage and fluid volume overload but also strongly predicts the likelihood of short-term death in individuals with acute heart failure [41]. The predictive effect of NT-proBNP level is consistent with the findings for prognosis in our meta-analysis. In addition, our meta-analysis failed to indicate a positive effect of ultrafiltration therapy on clinical prognoses such as all-cause mortality and rehospitalization, which was consistent with the results of previous meta-analyses [36, 37, 42] and clinical studies [10, 15, 43]. The absence of a correlation between prognosis and ultrafiltration therapy may be due to the possibility that ADHF is not a single disease but rather a family of related disorders, and thus the isolated ultrafiltration therapy during a short treatment time and follow-up could not substantially reverse cardiac remodeling or improve cardiac function. Hence, alleviating congestion in patients with ADHF using merely ultrafiltration or drug treatment may have a limited effect on the short-term and long-term outcomes of death and rehospitalization, indicating the possible necessity of complementary interventions of both ultrafiltration and a diuretic-based pharmacology strategy [35]. Notably, previous key studies have demonstrated that angiotensin-converting enzyme inhibition, beta-blockers, aldosterone receptor antagonists, and angiotensin-neprilysin inhibition are associated with long-term favorable effects on the clinical prognosis of death and remission [3, 6, 44–47]. Thus, standard and comprehensive treatment is required not only to relieve the symptoms and signs but also to reduce hospital admission and to increase survival.
This meta-analysis of 19 studies with a total of 1281 patients shows that ultrafiltration is superior to standard medical therapy (the main drugs included intravenous diuretics) for serum sodium removal, which is different from the conclusions of the meta-analyses of De Vecchis et al. [36], and Kwong et al. [37]. It is rational that ultrafiltration is superior in removing serum sodium, given the ultrafiltration mechanisms by which it provides an isotonic decongestion with an increased amount of sodium removal for a given fluid volume, whereas diuretics achieve decongestion through hypotonic fluid removal [35]. One of the pathophysiological signatures of ADHF is increased salt and water retention by the kidneys [48], and the effect of increased salt excretion by ultrafiltration seems to be beneficial for controlling the advance of ADHF. Despite the correlation between increased salt excretion and ADHF progress, more sodium removal does not mean better clinical outcomes, for hyponatremia in patients with heart failure can also reflect the severity, and the association between hyponatremia and adverse outcomes has been well established [49]. However, it is still unclear whether ultrafiltration has an impact on the incidence of hyponatremia, which may influence the prognosis of ADHF. More studies are needed to further verify the relationship between hyponatremia and ultrafiltration.
This meta-analysis indicates that ultrafiltration may be an effective method for fluid removal in patients with ADHF. However, taking the high cost, poor renal tolerability, and the attendant risks of venous access and heparinization into account, the use of ultrafiltration does not seem a conventional method for all ADHF patients at present and needs further development to improve its safety and effects on prognosis. More high-quality and well-designed RCTs in this field are needed, and consideration should be given to optimized drug treatment regimens, suitable rates and appropriate equipment for ultrafiltration, and the best time for initiating or discontinuing ultrafiltration therapy.
Study Limitations
Several limitations in the present meta-analysis should be considered. Firstly, the study design (nonblinded method), the baseline characteristics of the populations (consisting of the patients’ serum creatinine level at the baseline and higher left ventricular ejection fraction inclusion criteria), and the therapeutic regimen (ultrafiltration rate, diuretic species and dosage, and treatment duration) may give rise to heterogeneity and thus potentially affect our results. Secondly, our analysis was based on 19 RCTs with 1281 patients, and the sample sizes varied widely (from 12 to 224). Moreover, it is worth noting that some studies were of small scale and had relatively short follow-ups. Thirdly, mortality and rehospitalization are usually analyzed as time-to-event end points and the effects of treatment are commonly reported as hazard ratios. However, in the present meta-analysis, these end points were treated as binary variables because of failure to extract hazard ratios directly or to estimate them indirectly, possibly giving rise to potential bias of differing follow-up times between the trials. Finally, there was insufficient assessment of the impact of ultrafiltration versus diuretics on other meaningful clinical outcomes of cardiac structure and function, clinical symptoms, quality of life, and adverse events on account of scant reporting and the relatively short follow-up across the trials.
Conclusion
Compared with standard drug therapy, the use of ultrafiltration in ADHF patients is superior for volume management, but lacks superiority for prognosis. Moreover, the strategy of ultrafiltration has adverse renal effects, indicating that this approach to decongestion in ADHF patients is efficient for fluid management but less safe renally.








