Introduction
Coronary collateral circulation (CCC) is crucial and is related to the prognosis of patients with coronary artery disease. Well-developed CCC can restore blood flow to ischemic areas of the myocardium and protect myocardial tissue at risk [1]. Studies have shown that well-developed CCC can limit the area of myocardial infarction, reduce arrhythmia, protect cardiac function, reduce mortality, and ultimately improve the cardiovascular prognosis [2–4]. Currently, there is no noninvasive technology to evaluate collateral function [2, 5]. Therefore, the identification of circulating biomarkers to assess the advantages and disadvantages of CCC and exploration of its internal mechanism have important clinical significance.
Visfatin is an adipocyte factor that is produced in large quantities in visceral adipose tissue [6] and is additionally expressed within the heart, liver, muscle, placenta, lung, kidney, and bone marrow [7]. It has also been reported that visfatin plays a direct role in arterial remodeling [8]. Therefore, the connection between visfatin and CCC has become an area of interest, but research in this area is still lacking.
Vitamin D is a steroid hormone produced by irradiation of the skin with ultraviolet rays from the Sun. It performs biological functions by binding to vitamin D receptors, which are widely present in human tissues. Vitamin D receptors have been found on the surface of cardiomyocytes, smooth muscle cells, and endothelial cells, and control the proliferation and differentiation of these cells [9, 10].
Studies have shown that vitamin D in an independent factor that promotes proliferation and migration of human umbilical vein endothelial cells [11]. Additionally, increases in the number of myeloid cells [12] and regulation of the vascular endothelial growth factor (VEGF) signaling pathway [13] affect arteriogenesis and angiogenesis. Although evidence supports a positive regulatory role of vitamin D in both arteriogenesis and angiogenesis, few reports have examined its relationship with CCC in patients with chronic total occlusion (CTO).
In this study, we explored the relationship among the serum visfatin level, serum 25-hydroxyvitamin D3 [25(OH)D3] level, and CCC status in patients with CTO.
Methods
Patient Population
One hundred eighty-nine cases of at least one coronary artery with 100% diameter stenosis confirmed by coronary angiography were collected in the Department of Cardiology from November 2018 to February 2019 (100 men and 89 women; age 62±8 years). Patients were divided into a good CCC group (n=107, Rentrop grade 2 or 3) and a poor CCC group (n=82, Rentrop grade 0 or 1) according to the Rentrop-Cohen classification of CCC on coronary angiography.
The exclusion criteria included recent acute coronary syndrome (<3 months), coronary artery bypass operation, heart failure, cardiomyopathy, valvular heart disease, previous revascularization, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney or liver disease, previous diagnosis of malignancy, concomitant inflammatory disease, metabolic disease related to vitamin D, and the use of medications containing vitamin D preparations within the previous 3 months. Coronary artery disease risk factors such as hypertension, diabetes, hyperlipidemia, high body mass index, and smoking history were recorded for all patients.
The study was approved by the hospital ethics committee, and informed consent was obtained from each patient.
Coronary Angiography
Coronary angiography was performed by the Judkins method through radial artery puncture. CCC was graded according to the Rentrop-Cohen classification, as follows [14]: grade 0, no obvious collateral vessels (opaque area, no contrast medium filling in the distal infarction); grade 1, collateral perfusion of the coronary artery that did not reach the coronary artery of the pericardium; grade 2, partial perfusion of the subepithelial segment by CCC; and grade 3, perfusion of the coronary epicardial segment by CCC.
Biochemical Measurements
Fasting venous blood was drawn from the enrolled patients on the day of coronary angiography. Routine laboratory parameters, including the troponin I, creatinine, total cholesterol, triglyceride, low-density lipoprotein cholesterol (LDL-C), and calcium levels, were immediately determined. The blood samples collected for 25(OH)D3 and visfatin measurements were snap-frozen and stored at −70°C until analysis. The serum concentrations of 25(OH)D3 and visfatin were measured by the ELISA method with a Roche 601 analyzer (mlbio Linked Biotechnology Co., Ltd.).
Statistical Analysis
Statistical analysis was performed with the statistical package IBM SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA).
Continuous variables are expressed as the median and the 25th and 75th percentiles, categorical variables are expressed as numbers and percentages, and the chi-square test was used to assess the rational distribution hypothesis of continuous variables. Differences between two groups of normally distributed continuous variables were compared with a t test, and differences between non-normally distributed continuous variables were assessed by the Mann-Whitney U test. Categorical data were compared with the chi-square test, and Spearman’s correlation coefficient was used to assess the correlations between the serum 25(OH)D3 and visfatin levels and the Rentrop grade. Binary logistic regression analysis was used to determine independent predictors of poor CCC development. A P value of 0.05 or less was considered to indicate statistical significance.
Results
Baseline Patient Characteristics
The 189 patients included in the study were included in the good CCC group (good collaterals, 107 patients, Rentrop grade 2 or 3) or the poor CCC group (poor collaterals, 82 patients, Rentrop grade 0 or 1).
The baseline characteristics of the patients in the good CCC group and the poor CCC group are shown in Table 1. Both groups were similar in age, sex, body mass index, smoking history, presence of hypertension, and cholesterol and calcium levels (P>0.05). However, the proportion of patients with diabetes was higher in the poor CCC group than in the good CCC group (52.4% vs. 33.6%, P≤0.001). Furthermore, the LDL-C, troponin I, and creatinine levels were obviously higher in the poor CCC group than in the good CCC group, as shown in Table 1.
Demographic Properties and Biochemical Parameters of the Good Coronary Collateral Circulation (CCC) Group and the Poor CCC Group.
| Variable | Good (n = 107) | Poor (n = 82) | P |
|---|---|---|---|
| Age, years | 63 (56, 68) | 64 (58, 69) | 0.078 |
| Male sex, n (%) | 55 (51.4) | 45 (54.9) | 0.064 |
| Hypertension, n (%) | 57 (53.3) | 54 (65.9) | 0.082 |
| Diabetes, n (%) | 36 (33.6) | 43 (52.4) | 0.009 |
| Smoking history, n (%) | 55 (51.4) | 45 (54.9) | 0.635 |
| BMI, kg/m2 | 26 (25, 29) | 26 (26, 29) | 0.204 |
| LDL-C, mmol/L | 1.5 (0.65, 3.02) | 2.6 (2.03, 3.02) | <0.001 |
| TG, mmol/L | 2.2 (1.7, 2.6) | 2.03 (1.58, 2.08) | 0.15 |
| TC, mmol/L | 4.2 (3.8, 5.01) | 4.3 (3.9, 4.8) | 0.32 |
| TnI, ng/mL | 0.08 (0.05, 0.16) | 0.16 (0.12, 0.25) | <0.001 |
| Creatinine, mg/L | 66 (50, 90) | 75 (59, 90) | 0.034 |
| 25(OH)D3, ng/mL | 32 (29, 35) | 25 (21, 28) | <0.001 |
| Calcium, mmol/L | 2.06 (2.02, 2.1) | 2.06 (2.03, 2.1) | 0.182 |
| Visfatin, ng/mL | 10.3 (9.2, 12.8) | 17.4 (14.2, 21.13) | <0.001 |
| Rentrop score (n) | |||
| 0 | 18 | ||
| 1 | 64 | ||
| 2 | 64 | ||
| 3 | 43 |
Continuous variables are expressed the median and the 25th and 75th percentiles in parentheses, and categorical variables are expressed as the number and percentage.
BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; 25(OH)D3, 25-hydroxyvitamin D3; TC, total cholesterol; TG, triglycerides; TnI, troponin I.
Serum Visfatin and 25(OH)D3 Levels in Each Patient Group
The serum visfatin level was significantly higher in the poor CCC group than in the good CCC group (P<0.001), and the serum 25(OH)D3 level was significantly lower in the poor CCC group than in the good CCC group (P<0.001), as shown in Figures 1 and 2.
Relationship Among the Serum Visfatin and 25(OH)D3 Levels and the Rentrop Grade
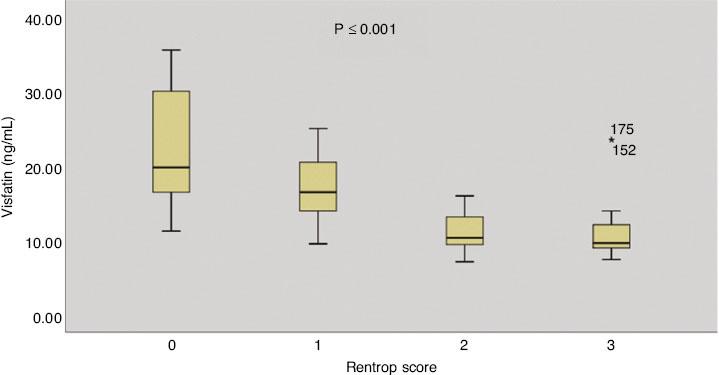
Spearman correlation analysis showed a significant negative correlation between the visfatin level and the Rentrop grade (r=−0.692, P<0.001) (Figure 3). A significant positive correlation was observed between the 25(OH)D3 level and the Rentrop grade (r=0.689, P<0.001) (Figure 4). Furthermore, an L-shaped relationship between the 25(OH)D3 and visfatin levels was observed (Figure 5).
Comparison of Visfatin Levels in the Good Coronary Collateral Circulation (CCC) Group and the Poor CCC Group.
Binary Logistic Regression Analysis
Binary logistic regression was performed between CCC development and 25(OH) D3, visfatin, and LDL-C levels and other factors. CCC development was used as the dependent variable, and all univariate analyses with P values less than 0.1 were included in the logistic regression equation as independent variables. Stepwise regression was used for multivariate analysis. The serum 25(OH)D3, visfatin, and LDL-C levels and diabetes were independent risk factors for poor CCC development (Figure 6).
Discussion
The present study revealed the following findings: (1) The visfatin level was significantly higher in patients with CTO in the poor CCC group than in those in the good CCC group, and a positive correlation was observed between a high visfatin level and poorly developed CCC. A high visfatin level was an independent predictor of poor CCC in patients with CTO. (2) The 25(OH)D3 level was significantly lower in patients with CTO in the poor CCC group than in patients with CTO in the good CCC group. A low serum 25(OH)D3 level was positively correlated with poorly developed CCC in patients with CTO and was an independent predictor of poorly developed CCC in patients with CTO. (3) An L-shaped relationship between the serum 25(OH)D3 and visfatin levels was observed. (4) A high LDL-C level and a history of diabetes also contributed to poor CCC development in patients with CTO.
Coronary collateral growth is thought to develop by the expansion of a preexisting collateral network and the formation of new blood vessels [15, 16]. Recent studies have found that the main factor affecting the development of coronary collateral vessels is the pressure gradient between segments located at the proximal and distal ends of the occlusion [17, 18]. VEGF, endothelial progenitor cells (EPCs), fibroblast growth factor, transforming growth factorβ, and nitric oxide (NO)-activated biological substances also play significant roles in both angiogenesis and arteriogenesis [19].
Visfatin is a known proinflammatory cytokine that plays an important role in many chronic inflammatory diseases, including atherosclerosis and cardiovascular diseases. As a cytokine, visfatin can regulate the expression of key regulators of vascular remodelling, such as VEGF [20], fibroblast growth factor 2 [21], and matrix metalloproteinases [22]. Recent studies showed that visfatin promotes angiogenesis through a VEGF-dependent mechanism in EPCs [23]. Auguet et al. [24] found that visfatin was highly expressed in unstable carotid plaques and coronary atherosclerotic plaques of lipomacrophages. In addition, it was recently reported that extracellular visfatin is directly involved in vascular remodeling [8]. However, the relationship between visfatin and CCC is still unclear.
This study reports, for the first time, the relationship between visfatin and CCC in patients with CTO. The visfatin level was significantly higher in patients with CTO in the poor CCC group than in patients with CTO in the good CCC group, which may be related to the following mechanisms: (1) Visfatin induces oxidized low-density lipoprotein receptor1 expression in vascular endothelial cells, which causes inflammation, leads to endothelial cell damage and dysfunction, and promotes the vicious cycle of atherosclerosis [25]. (2) Visfatin activates extracellular-signal-regulated kinase 1/2 and nuclear factorκB, resulting in increased production of inducible nitric oxide synthase [26]. Inducible nitric oxide synthase is a proinflammatory enzyme that can cause an imbalance in NO production, induce endothelial dysfunction, and ultimately lead to impaired angiogenesis. Therefore, the inflammatory effect of visfatin promotes endothelial dysfunction, which may result in poor CCC development. Visfatin also has a two-way regulatory effect in metabolic diseases. Further research is needed to understand the impact of visfatin in different situations and clinical conditions related to cardiovascular disease.
Recent studies have shown that vitamin D can regulate the growth of endothelial cells and smooth muscle [27], and vitamin D deficiency is positively correlated with the degree of coronary vascular stenosis [28]. Vitamin D is also closely related to the degree of vascular endothelial dysfunction in patients with coronary heart disease [29]. However, the relationship between vitamin D and CCC and the underlying mechanism are largely unclear.
In our study, the 25(OH)D3 level was significantly higher in the good CCC group than in the poor CCC group. This is consistent with the results reported by Dogan et al. [30]. The following points may provide a reasonable explanation for the poor CCC development caused by vitamin D deficiency: (1) Vitamin D promotes the adhesion of white blood cells, increases VEGF-A expression, and promotes the growth and migration of vascular smooth muscle cells [31, 32]; therefore, vitamin D deficiency can lead to impaired CCC development. (2) Vitamin D activates and induces NO production, mediates the VEGF signaling pathway, and stimulates the growth of coronary collaterals [33, 34], leading to poor CCC development. Cianciolo et al. [35] showed that vitamin D receptors are also present on circulating EPCs. Vitamin D deficiency can not only reduce the number of circulating EPCs but can also decrease their ability to proliferate and form blood vessels, leading to impaired CCC development.
Our study is also the first to show an L-shaped relationship between 25(OH)D3 and visfatin levels. In the absence of 25(OH)D3, when the visfatin level was less than 30 nmol/L, a negative correlation was observed between 25(OH)D3 and visfatin levels. However, when the 25(OH)D3 level was greater than 30 nmol/L, the visfatin level did not continue to decrease with increasing 25(OH)D3 level; in contrast, the level remained constant. This phenomenon may suggest that supplementation with vitaminD to the normal range will not lead to further declines in the levels of endolipids in patients with vitamin D deficiency. This may also provide another possible explanation for the results reported by Luttmann-Gibson et al. [36], indicating an absence of cardiovascular benefits from vitamin supplementation (it is worth noting that in the intervention and control groups, the plasma 25(OH)D3 levels at the baseline and 12 months after the intervention were higher than 50 nmol/L).
Our study found that the serum LDL-C level was significantly higher in the poor CCC group than in the good CCC group. Moreover, the 25(OH)D3 level was negatively correlated with the LDL-C level, which is similar to the results reported by Song et al. [37].
High levels of LDL-C are a risk factor for coronary artery disease, and can induce endothelial cell dysfunction and impair the growth of coronary collateral vessels [38, 39].
In our study, the frequency of diabetes was significantly higher in the poor CCC group (52.4%) than in the good CCC group (33.6%), and patients with diabetes had lower vitamin D levels than patients without diabetes. Nisanci et al. [40] also found insufficient opening of the CCC in patients with diabetes by measuring the coronary wedge pressure. Studies have found that because of the severely impaired function of vascular endothelial cells in patients with diabetes, the abilities of these cells to proliferate, adhere, integrate, and eventually undergo angiogenesis are significantly reduced [41].
In conclusion, visfatin and vitamin D seem to play important roles in the regulation of key mechanisms involved in CCC development.
Seasonal changes in vitamin D levels and exposure to the Sun may affect the results of the study, so we recruited patients from November to February to minimize interindividual variability. In addition, physical exercise is also one of the factors that affects the level of vitamin D and the development of CCC. Although the patients we selected indicated that they did not participate in regular physical exercise, this could not completely eliminate the impact on the results of the study. This is the shortcoming of this study.






