Introduction
Percutaneous coronary intervention (PCI) remains one of the most minimally invasive procedures to treat coronary artery disease [1]. However, it may also expose interventional cardiologists to ionizing radiation and place them at risk of orthopedic injuries. The development of robotic assisted PCI could protect surgeons against such occupational hazards, and increase the precision of interventional procedures [2]. The first-generation remote navigation system (RNS) (Navicath, Haifa, Israel) was developed in 2006 [3]. The system consisted of ① a remote control cockpit, ② an articulated robotic arm attached to the catheterization table, ③ and a disposable cassette to which the guide catheter was connected. A key component of the control cockpit was a tabletop motorized drive, called a joystick, used to advance and retrieve the intravascular devices. The second generation robotic PCI system, CorPath 200 System (Corindus Vascular Robotics, Natick, MA, USA), was developed in 2011 [4] and had a control system similar to the RNS. The control cockpit incorporated a touchscreen control console, and two joysticks to control the guidewire and balloon/stent. Surgeons were able to control the cassette to perform linear and rotational motions via the joysticks, and could perform discrete movements via the touch screen. Cabling connected the control cockpit to the robotic arm. The third generation robotic PCI system, Corindus CorPath GRX, was developed in 2018 [5]. Advances incorporated in the CorPath 200 included the addition of a third joystick with the ability to remotely control the guide catheter and the addition of “rotate-on-retract” imitation to help control the guidewire [6].
Joystick control systems have several intrinsic limitations. First, the surgeon’s experience in conventional PCI does not directly translate into experience in robotic PCI. Second, a control system such as a joystick cannot perform linear and rotational motions simultaneously, yet such control is important in passing complicated lesions, such as chronic total obstructions. Third, current systems lack direct force feedback allowing operators to determine the resistance of the guidewire. To overcome this problem, we developed a novel master-slave robotic control system to assist in PCI. We evaluated the safety and feasibility of delivery and manipulation of coronary guidewires in vitro and in vivo with this system.
Methods
Device Description
An overview of the setup of the novel robotic assisted PCI system is shown in Figure 1B. In our robotic PCI system, we sought to overcome the limitations of current control systems. We used a master-slave operation mode; i.e., a teleoperation system. During operation, the slave actuator (robotic arm and cassette) is arranged in the operation room under X-ray exposure, while the surgeon remains in the control room away from the X-ray and operates the master actuator (remote controller) to remotely control the slave actuator (Figure 2E). The communication system in the control room receives operating information from the master actuator and transmits the information to the slave actuator through wireless internet; feedback information can also be received from the slave actuator and transmitted to the master actuator (Figure 1B). In robotic systems, problems such as communication delays and uncertainties must be properly addressed. To eliminate communication delay and uncertainty, the transmission mechanism was greatly simplified by removing the gear and synchronous belt transmission, so that the motor shaft was directly connected to the guidewire clamp in the slave actuator.
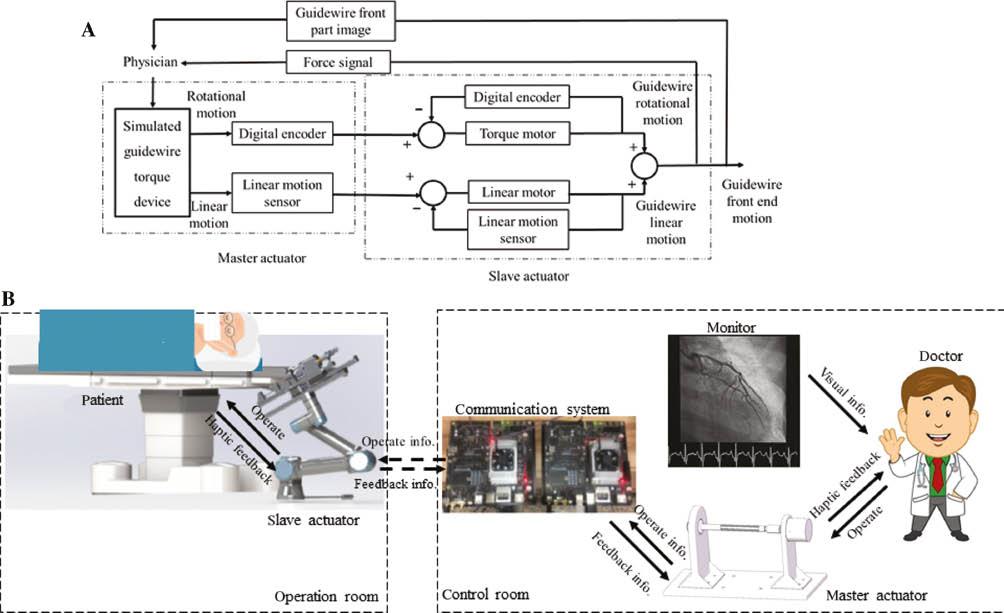
Setup of the Novel Robotic Assisted PCI System.
(A) Control of a guidewire by the novel robotic control system. A surgeon uses the simulated guidewire torque device to remotely control the linear and rotational (torque) motion of guidewire, under the guidance of haptic and visual feedback. (B) Representative system structure. The system is composed of three parts: the master actuator, which imitates the traditional torque used by surgeons in conventional PCI; the slave actuator, which includes a guidewire delivery system and force monitoring equipment; and a communication system, which connects the master and slave actuators.
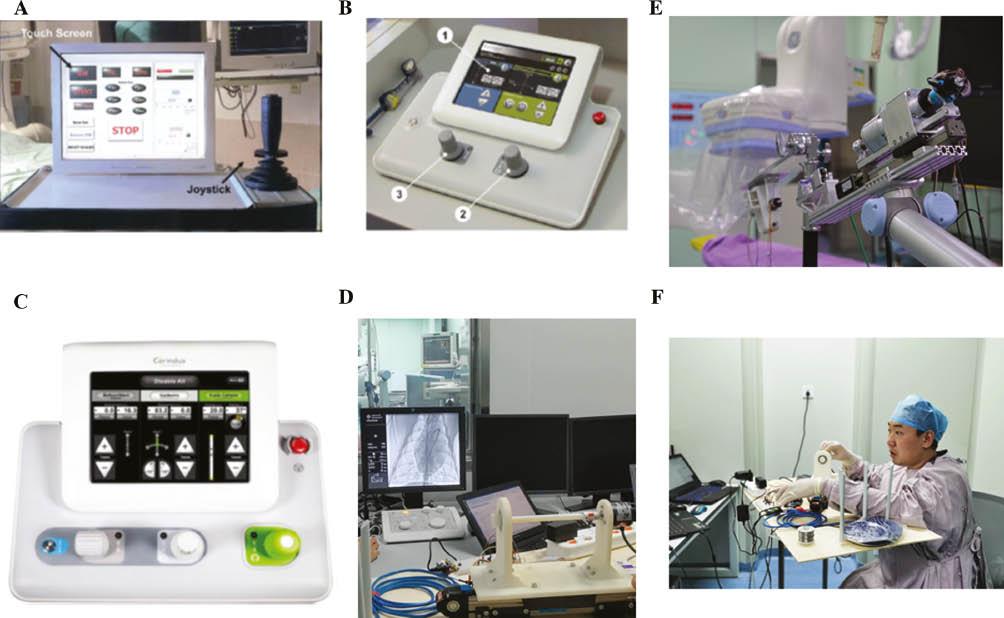
Comparison of the Novel Control System with Current Robotic-Assisted PCI Control Systems.
(A) The remote control panel, including the joystick of the RNS system. (B) Representative view from within the radiation-shielded remote control cockpit of the CorPath 200 system [4]. (C) Remote control panel of the Corindus CorPath GRX system [5]. (D) Remote control panel of the novel robotic assisted PCI system, which imitates the traditional torque used by surgeons in conventional PCI. (E) Slave actuator of the novel robotic assisted PCI system. (F) Surgeon operating the master actuator of the novel robotic assisted PCI system.
Unique Design of the Master Actuator
In conventional PCI, surgeons operate the guidewire through torque via pushing and rotating, guided by X-ray images. To avoid long training times and make use of surgeons’ prior experience, the master actuator is designed to imitate the traditional torque used by surgeons in conventional PCI (Figure 2D). The master actuator is fixed on a sliding block connected to a slide rail, thus providing linear and rotation motion simultaneously, reflecting the system’s two degrees of freedom (Figure 1A).
Unique Design of the Slave Actuator
The slave actuator system includes a mechanical arm, a guidewire clip and manipulator, and force monitoring equipment. The design mimics surgeons’ pushing and rotating of the guidewire in conventional PCI. The guidewire clip and manipulator are designed to imitate the traditional torque used by surgeons in conventional PCI (Figure 3).
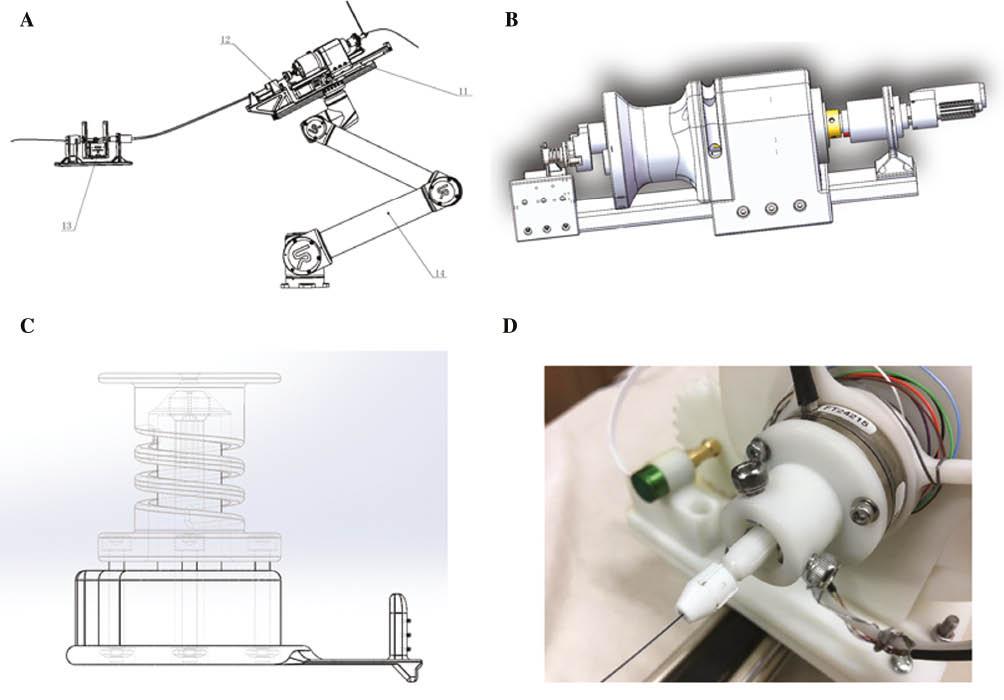
Design of the Guidewire Delivery System.
(A) The guidewire delivery system includes a mechanical arm, a guidewire clip and manipulator, and force monitoring equipment. (B) Three-dimensional external form of the guidewire clip and manipulator. (C) Schematic design of the guidewire clip and manipulator. (D) Physical map of the guidewire clip and manipulator.
Force Feedback Design
To further enhance the information communication, the master actuator is directly connected to the digital encoder and sensor. To sense the force along the guidewire, and to be compatible with conventional guidewires, the force sensor is installed at the side of a guidewire clamp to measure the force feedback (haptic feedback). However, the guidewire’s properties of high flexibility and multiple contacts with the vessel wall tend to result in incorrect force feedback information. Therefore, the guidance of the guidewire’s motion also largely relies on X-ray images, through a combination of haptic and visual feedback.
In vivo Experiment
The system was tested in anesthetized swine. Experiments were performed in six Bama minipigs (male, 18 months old, 20–25 kg weight). The study was approved by the ethics committee of Beijing Tsinghua Changgung Hospital (21245-7-01). The animals were randomized into either a robotic or a manual control group. Each animal was placed on the table of a monoplane catheter laboratory in supine position, and general anesthesia and mechanical ventilation were established. A 6-French (Fr) sheath was manually introduced into the right femoral artery for coronary access. Anticoagulation was established with unfractionated heparin (70 units per kilogram body weight) to avoid thrombus formation during the procedure. Angiography of both coronary arteries was performed with standard 6-Fr Judkins guiding catheters. A standard floppy-tip guidewire (Runthrough NS Extra Floppy®, TERUMO, Tokyo, Japan) was carefully advanced into distal branches of the right and left coronary arteries under robotic or manual control by the same experienced interventional cardiologist (Supplemental Video 2).
Results
In vitro Experiment
The system was initially tested on a transparent glass coronary model. The model demonstrated that the guidewire could be easily manipulated through vascular branches and positioned at the required location (Supplemental Video 1).
In vivo Experiment
A total of six pigs were randomized into either the robotic or the manual control group. Coronary angiography indicated no stenosis more than 50% in the left and right coronary arteries in all six pigs. An experienced interventional cardiologist who had no experience in operating a robotic PCI system advanced the guidewires into distal branches of the right and left coronary arteries by using the novel robotic PCI system, while sitting in the control room with no lead apron protection (Supplemental Video 2). The same cardiologist performed the same procedure manually at the catheterization table with lead apron protection. The radiation exposure and operation times were recorded. Both robotic and manual control resulted in completion of the operation with no device- or procedure-associated complications. As shown in Table 1, the first-time user of the novel robotic PCI system required similar times to advance the guidewire into distal branches of coronary arteries (13.23±3.55s vs. 11.19±4.58s, P=0.45) with the PCI system and the manual procedure. The operator radiation exposure in the robotic assisted procedure was 99.99% lower than that in the manual procedure (<0.25 μGy vs. 15.32±8.87 μGy; P < 0.001). The operator rated the robotic system performance as equal to that of the manual procedure. All procedures performed by the robotic system were completed without any periprocedural complications.
Discussion
Robotics in catheterization laboratories has been applied to PCI for approximately 15 years, with the aim of decreasing occupational hazards [7]. However, using the current joystick based operation systems differs from performing conventional PCI; therefore, surgeons’ experience cannot directly translate to robotic PCI, and learning times are relatively long. In the PRECISE multi-center robotic-enhanced PCI study, the procedural duration and fluoroscopy time became significantly shorter after 60 operations were performed [8]. Here, we designed a novel robotic assisted PCI system to mimic surgeons’ pushing and rotating of the guidewire in conventional PCI. The guidewire clip and manipulator were designed to imitate the traditional torque used by surgeons in conventional PCI. To our knowledge, such a system has not previously been reported. With the novel robotic assisted PCI system, a first-time user with no prior experience in operating robotic PCI systems required similar times to advance the guidewire with the PCI system and the manual procedure. These comparable results were attributed to the unique design of the novel control system, which imitates the traditional torque used by surgeons in conventional PCI.
Force feedback is another important feature to enhance the accuracy and safety of robotic assisted PCI [9, 10]. However, current systems lack direct force feedback to indicate the resistance of the guidewire to the operator. In contrast, our novel robotic assisted PCI system combines direct force feedback from a force sensor at the side of guidewire clamp with visual feedback from X-ray images to help direct the guidewire’s motion. Surgeons have found that this unique design greatly enhances the accuracy and safety of robotic assisted PCI.
For better interpretation of the results, some limitations in this study should be acknowledged. First, the results were derived from a swine model, which slightly differs from humans. Therefore, the current results must be verified in humans. Second, our experiment must be repeated in pigs with more than 70% coronary stenosis or complicated lesions, because such lesions are associated with longer operation times and higher radiation exposure [11]. Additionally, we investigated only the control of the guidewire; thus, control of the guide-catheter, balloon, and stent must be reported in future work. Finally, given that we investigated only the short-term effects of robotic PCI, long-term effects remain to be evaluated.
In conclusion, our early in vivo experience with the novel robotic assisted PCI control system demonstrated that its feasibility, safety, and procedural effectiveness are comparable to those of manual operation, and that it requires significantly shorter learning times than other currently available systems. A larger in vivo study and subsequent clinical studies are warranted to verify the safety and effectiveness of this system.

