Significance Statement
The present study is the first to assess the risk of severe AAC according to uric NNN, the dose–response relationship between uric NNN and severe AAC, and the threshold effect.
Our findings may promote public understanding of the harm of cigarette smoking and the benefits of cigarette smoking cessation, because smoking cigarettes releases at least 4000 molecular constituents.
Uric NNN may also be a potential biomarker to quantitatively evaluate tobacco exposure and the effects of cigarette smoking cessation.
Future basic, observational, and interventional studies will be valuable for cardiovascular disease prevention and the identification of new therapeutic targets.
Abbreviations and Acronyms: AAC, abdominal aortic calcification; BMI, body mass index; CI, confidence interval; CS, cigarette smoking; CVDs, cardiovascular diseases; DM, diabetes mellitus; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N′-nitrosonornicotine; OR, odds ratio; SD, standard deviation; SP, serum phosphorus; STC, serum total calcium; STROBE, STrengthening the Reporting of OBservational studies in Epidemiology; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides; TSNA, tobacco-specific nitrosamine; US, United States.
Introduction
Epidemiological studies have strongly suggested that cigarette smoking (CS) is a major modifiable risk factor for the incidence and mortality of cardiovascular disease (CVD) [1–4]. The potential mechanism involves aggravated inflammation, oxidative stress, and thrombosis [5–7]. The dose-dependent correlation between CS and CVD risk remains unclear, because of the difficulty in measuring the extent of tobacco use and exposure to environmental tobacco smoke [8–10]. Cigarettes release at least 4000 molecular constituents, including tobacco-specific nitrosamines (TSNAs), which are well recognized as a leading class of carcinogens [11]. Because of their specificity for tobacco, TSNAs may serve as biomarkers for tobacco carcinogen uptake. Among different types of TSNAs, N′-nitrosonornicotine (NNN) is noteworthy for its associations with lung, oral cavity, and esophageal cancers in tobacco users [12–14]. In addition, the abundance of NNN is markedly higher than that of other TSNAs [15]. However, the relationship between NNN and CVD had not been investigated.
Many recent studies have reported associations among dietary data, laboratory indices, and abdominal aortic calcification (AAC), on the basis of data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 [16–23]. AAC has been widely discussed because it is an important predictor of CVD mortality [24–28], and it can be noninvasively and precisely examined in hospital settings [29–32]. However, the relationship between TSNAs and AAC remains unclear. This study is the first cross-sectional study to explore the dose–response relationship between uric NNN levels and severe AAC in a noninstitutionalized United States (US) civilian population. We aimed to identify a reliable biomarker reflecting exposure to both mainstream and environmental tobacco smoke, and its correlation with CVD risk.
Methods
Ethics
The study protocol was approved by the Ethics Review Board of the National Center for Health Statistics. All participants signed an informed consent form before participating in the study. All data in the present study are available to the public at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013. The ethics committee approval number of NHANES 2013–2014 is National Center for Health Statistics Institutional Review Board/Ethics Review Board protocol number 2011–17, which can be found at https://www.cdc.gov/nchs/nhanes/irba98.html. The entire article is organized according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Study Population and Design
The present study was performed by using data from NHANES 2013–2014. NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the US. It is a major program of the National Center for Health Statistics, part of the Centers for Disease Control and Prevention. In NHANES 2013–2014, 10,175 individuals were involved in the interviews, and AAC was evaluated in people 40 years of age or older. We initially included 7910 participants with uric NNN information. We then excluded 5038 participants with missing AAC scores and 159 participants who did not consume alcohol. Finally, a total of 2713 participants were included in this study. A detailed flowchart of the participant recruitment process is shown in Figure 1.
Data Collection and Measurements
As previously documented [18], demographics, health conditions, and lifestyle data were derived from household interview questionnaires and mobile examination center questionnaires administered by trained interviewers. The demographic data included age, sex, and race/ethnicity. Race/ethnicity included Mexican American, other Hispanic, non-Hispanic white, non-Hispanic Black, and other races/ethnicities. We merged Mexican Americans and other Hispanics into one group (Hispanic), and merged non-Hispanic white, non-Hispanic Black, and other races/ethnicities into another (non-Hispanic) in the subgroup analysis (Figure 2), as previously reported [18]. The health condition data comprised hypertension and diabetes mellitus (DM) history, which was examined through the item “doctor or other health professional told you have DM and/or high blood pressure.” The alcohol intake was assessed on the basis of the item “had at least 12 alcohol drinks per year.” Body mass index (BMI) was expressed as weight in kilograms divided by height in meters squared and was obtained from the NHANES body measurement data.
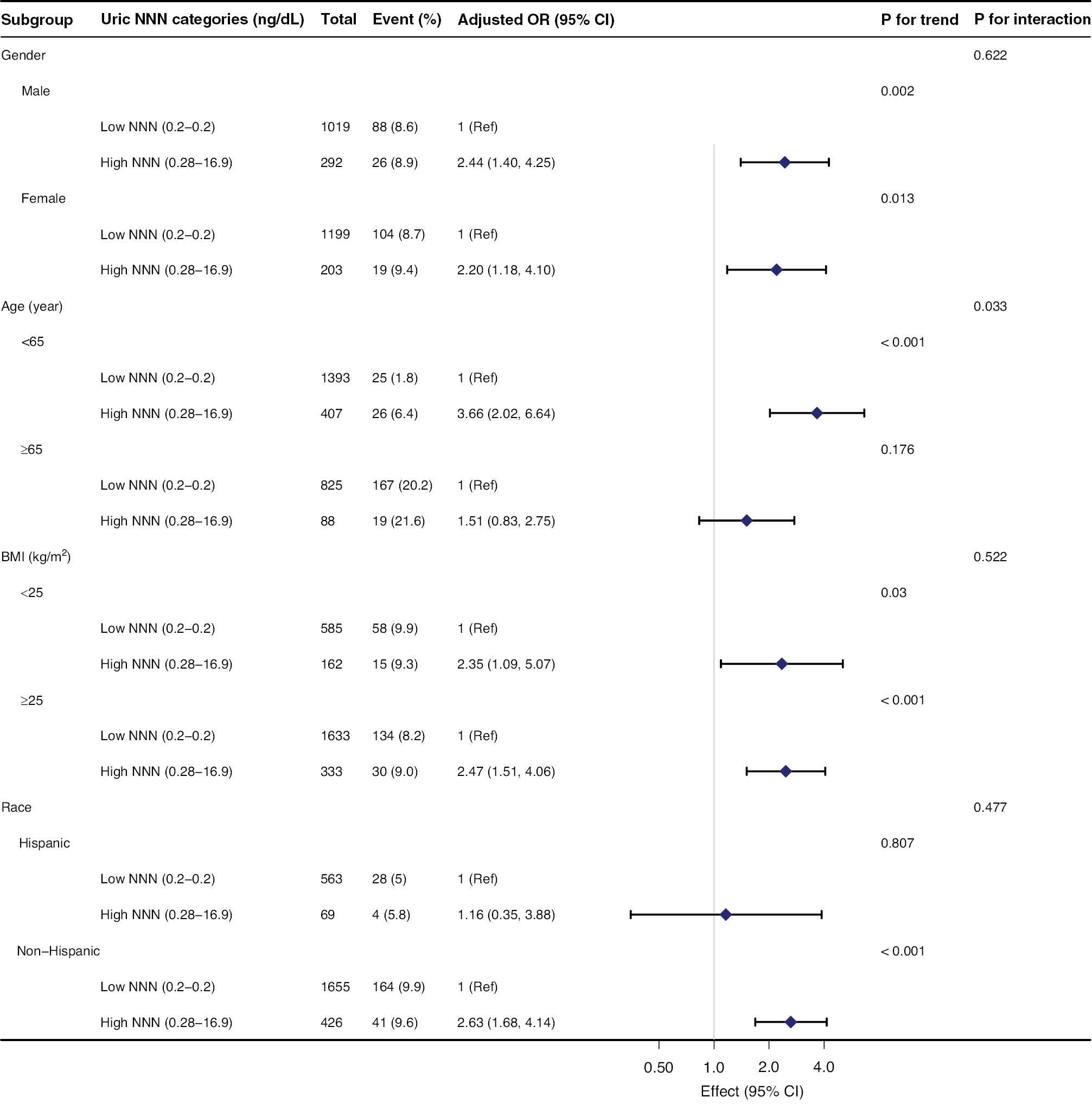
Subgroup Analyses of the Association Between Uric NNN Levels and Severe AAC in the NHANES 2013–2014 Database.
Adjustments were made for age, sex, race, body mass index, alcohol intake, hypertension, diabetes mellitus, total cholesterol, triglycerides, serum uric acid, serum total calcium, serum phosphorus, serum creatinine, and total 25-hydroxyvitamin D. AAC, abdominal aortic calcification. NHANES, National Health and Nutrition Examination Survey. OR, odds ratio. CI, confidence interval. BMI, body mass index. NNN, N′-nitrosonornicotine.
A Beckman UniCel DxC800 Synchron System (Beckman, Fullerton, CA, USA) was used to measure the serum total cholesterol (TC), triglycerides (TG), and serum uric acid (SUA) through a timed-endpoint method; serum total calcium (STC) through indirect (or diluted) ion selective electrode methods; serum phosphorus (SP) through a timed-rate method; and serum creatinine through the Jaffe rate method (kinetic alkaline picrate). The 25-hydroxyvitamin D levels were measured with standardized liquid chromatography-tandem mass spectrometry. Detailed information on the laboratory tests is available at https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2013.
Uric NNN Examination
Urinary TSNAs were measured with isotope-diluted high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. More details are available at the official website: https://wwwn.cdc.gov/Nchs/Nhanes/2013–2014/TSNA_H.htm.
AAC Evaluation
As previously reported [18, 20, 22, 23], AAC was determined by a lateral scan of the lumbar spine (vertebrae L1–L4) with dual-energy X-ray absorptiometry. The Kauppila score system was applied to quantify the extent of AAC [20, 22, 23, 31]. More details are available at the official website: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DXXAAC_H.htm. The AAC score ranged from 0 to 24, and scores >6 were defined as severe AAC, on the basis of previous studies [18, 20, 30, 32].
Statistical Analysis
Continuous variables are shown as mean ± standard deviation or median (interquartile range, IQR). Categorical variables are presented as frequency or percentage (n, %). The normality of the distribution was confirmed with the Shapiro–Wilk test. Uric NNN levels were analyzed as categorical variables, and the low NNN group was defined as the reference. For analysis of baseline characteristics, the statistical differences among groups of uric NNN were tested with one-way analysis of variance for continuous variables and chi-square test for categorical variables. The odds ratios (ORs) and 95% confidence intervals (CIs) for severe AAC in the uric NNN category were determined with multivariate logistic regression models. Both the crude and adjusted models (models I–III) are shown. The potential covariates included age, sex, race, BMI, alcohol intake, hypertension, DM, TC, TG, SUA, STC, SP, total 25-hydroxyvitamin D, and serum creatinine levels. Trends were estimated with linear regression by entering the median value of each uric NNN group as a continuous variable in the models.
A generalized additive model was used to assess the nonlinear relationship between uric NNN levels and the risk of severe AAC. Nonlinearity was tested with a cubic spline term. Considering the smoothing curve, a two-piecewise linear regression model was designed to determine the threshold effect with adjustment for potential confounders. The threshold level of the uric NNN was determined with a recurrence method that included selecting the turning point along a predefined interval and choosing the turning point that yielded the maximum likelihood model. A likelihood ratio test was used to compare the two-piecewise linear regression model with the one-line linear model. Subgroup analyses were performed with stratified logistic regression models. Interactions across the subgroups were tested with a likelihood ratio test.
All statistical analyses were performed with the statistical package R (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.8. The packages of tableone, rms, ggplot2, and forestplot were used for statistical analyses. Statistical significance was defined as a two-sided P value <0.05.
Results
The database is presented in Supplementary Table 1. The demographic characteristics of the 2713 participants (1311 men and 1402 women), stratified by uric NNN levels are presented in Table 1. The mean age was 59.0 ± 11.9 years, and the median (IQR) uric NNN level was 0.2 (0.2, 0.2) ng/dL. Because of the lack of a well-established standard, the uric NNN level was categorized as low or high through the median concentration (0.2 ng/dL) in the present study. Overall, individuals with high uric NNN levels were younger and more likely to be men, to have a lower BMI, to have a habit of alcohol intake, and to have lower levels of serum total 25-hydroxyvitamin D than individuals with low uric NNN levels (all P < 0.05).
Baseline Characteristics of NHANES 2013–2014 Participants by Uric NNN Level.
| Variable | All participants | Uric NNN level (ng/dL) | P value | |
|---|---|---|---|---|
| Low NNN (0.2–0.2) | High NNN (0.28–16.9) | |||
| Participants (n) | 2713 | 2218 | 495 | |
| Uric NNN (ng/dL) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 1.2 (0.7, 2.3) | < 0.001 |
| AAC prevalence (n, %) | 810 (29.9) | 650 (29.3) | 160 (32.3) | 0.185 |
| Age (years) | 59.0 ± 11.9 | 59.8 ± 12.1 | 55.2 ± 9.7 | < 0.001 |
| Male (n, %) | 1311 (48.3) | 1019 (45.9) | 292 (59) | < 0.001 |
| BMI (kg/m2) | 28.5 ± 5.6 | 28.7 ± 5.5 | 27.8 ± 5.8 | 0.001 |
| Race (n, %) | < 0.001 | |||
| Mexican American; | 362 (13.3) | 329 (14.8) | 33 (6.7) | |
| Other Hispanic | 270 (10.0) | 234 (10.6) | 36 (7.3) | |
| Non-Hispanic White | 1217 (44.9) | 965 (43.5) | 252 (50.9) | |
| Non-Hispanic Black | 509 (18.8) | 384 (17.3) | 125 (25.3) | |
| Other Race | 355 (13.1) | 306 (13.8) | 49 (9.9) | |
| Alcohol intake (at least 12 alcohol drinks per year) (n, %) | 1943 (71.6) | 1523 (68.7) | 420 (84.8) | < 0.001 |
| Hypertension (n, %) | 1286 (47.4) | 1063 (47.9) | 223 (45.1) | 0.247 |
| Diabetes Mellitus (n, %) | 446 (16.4) | 379 (17.1) | 67 (13.5) | 0.054 |
| Total Cholesterol (mmol/L) | 5.1 ± 1.1 | 5.1 ± 1.1 | 5.1 ± 1.1 | 0.750 |
| Triglycerides (mmol/L) | 1.4 (0.9, 2.2) | 1.4 (0.9, 2.2) | 1.5 (1.0, 2.4) | 0.059 |
| Serum uric acid (μmol/L) | 324.5 ± 81.8 | 324.4 ± 81.1 | 324.9 ± 85.0 | 0.894 |
| Serum total calcium (mmol/L) | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 0.976 |
| Serum phosphorus (mmol/L) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.294 |
| Total 25-hydroxyvitamin D (nmol/L) | 71.0 ± 29.5 | 72.8 ± 29.4 | 63.0 ± 28.3 | < 0.001 |
| Serum creatinine (μmol/L) | 77.8 (65.4, 91.9) | 76.9 (65.4, 91.9) | 79.6 (67.2, 91.0) | 0.093 |
Data are shown as mean ± SD, median (IQR), or n (%). Abbreviations: AAC, abdominal aortic calcification; BMI, body mass index; NNN, N′-nitrosonornicotine.
The associations between uric NNN levels and severe AAC are shown in Table 2. The prevalence of severe AAC was 8.7% (n = 237). In all adjusted models (models I–III), but not in the crude model, the risk of severe AAC was greater in the high uric NNN group than the low uric NNN group. In the fully adjusted model (model III, adjustment for age, sex, race, BMI, alcohol intake, hypertension, DM, TC, TG, SUA, STC, SP, total 25-hydroxyvitamin D, and serum creatinine), the adjusted OR for participants in the high uric NNN group versus the low uric NNN group was 2.39 (95% CI: 1.59, 3.61; P < 0.001).
Association Between Uric NNN Levels and Severe AAC in the Database from NHANES 2013–2014.
| Uric NNN categories (ng/dL) | Number of participants | Severe AAC (n, %) | Crude model OR (95% CI) | Model I OR (95% CI) | Model II OR (95% CI) | Model III OR (95% CI) |
|---|---|---|---|---|---|---|
| 2713 | 237 (8.7) | |||||
| Low NNN (0.2-0.2) | 2218 | 192 (8.7) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| High NNN (0.28-16.9) | 495 | 45 (9.1) | 1.06 (0.75, 1.48) | 2.53 (1.70, 3.75) | 2.42 (1.61, 3.62) | 2.39 (1.59, 3.61) |
| P value | 0.757 | < 0.001 | < 0.001 | < 0.001 |
Model I adjusts for age, sex and race; model II adjusts for model I + body mass index, alcohol intake, hypertension, and diabetes mellitus; model III adjusts for model II + total cholesterol, triglycerides, serum uric acid, serum total calcium, serum phosphorus, serum creatinine, and total 25-hydroxyvitamin D. NNN, N′-nitrosonornicotine. AAC, abdominal aortic calcification. NHANES, National Health and Nutrition Examination Survey. OR, odds ratio. CI, confidence interval. Ref, reference.
The dose–response relationship between uric NNN and severe AAC is shown in Figure 3 with a restricted cubic spline model (P for nonlinear = 0.025) after adjustment for age, sex, race, BMI, alcohol intake, hypertension, DM, TC, TG, SUA, STC, SP, total 25-hydroxyvitamin D, and serum creatinine levels. To evaluate the threshold effect of uric NNN levels on severe AAC, after adjustment for the confounding factors described above, we developed a two-piecewise linear regression model according to the smoothing curve shown in Figure 3. As shown in Table 3, the risk of severe AAC was positively correlated with uric NNN levels until 1.354 ng/dL (95% CI: 1.201–1.507), and the OR was 2.515 (95% CI: 1.100–5.748, P = 0.029). When the uric NNN level exceeded 1.354 ng/dL, the OR was 0.798 (95% CI: 0.459–1.387, P = 0.423), thus suggesting that the risk of severe AAC did not increase significantly with a further increase in uric NNN levels (P = 0.017 for the likelihood ratio test) (Table 3 and Figure 3).
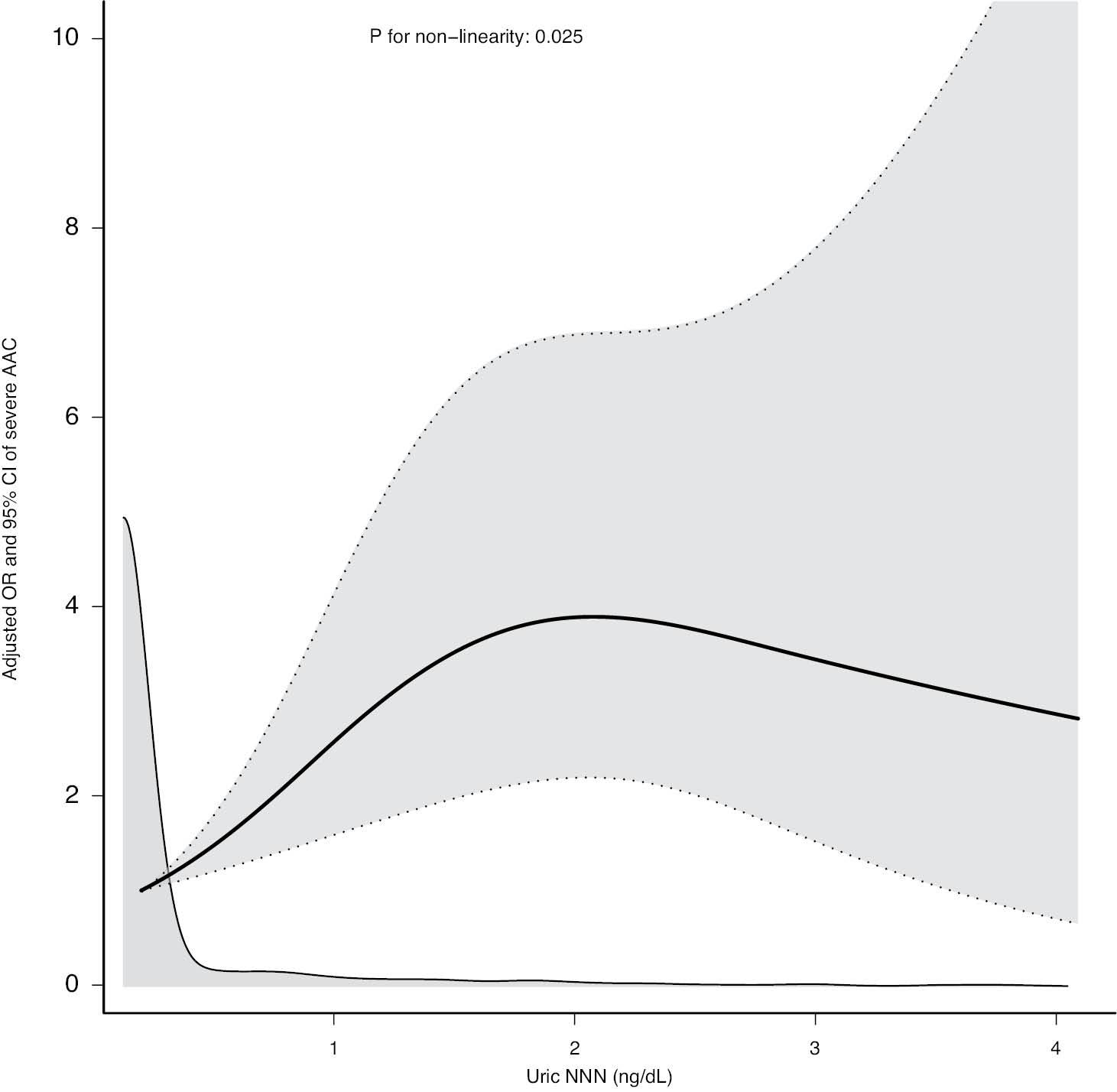
Relationship between Uric NNN Levels and Severe AAC in the NHANES 2013–2014 Database.
The relationship was demonstrated through a cubic spline model after adjustment for potential confounding factors (P for nonlinear = 0.025). Four knots were chosen. Adjustments were made for age, sex, race, body mass index, alcohol intake, hypertension, diabetes mellitus, total cholesterol, triglycerides, serum uric acid, serum total calcium, serum phosphorus, serum creatinine, and total 25-hydroxyvitamin D. AAC, abdominal aortic calcification. NHANES, National Health and Nutrition Examination Survey. OR, odds ratio. CI, confidence interval. NNN, N′-nitrosonornicotine.
Threshold Effect Analysis of Uric NNN Levels on Severe AAC in the NHANES 2013–2014 Database.
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Two-piecewise linear regression model | ||
| Uric NNN <1.354 ng/dL * | 2.515 (1.100, 5.748) | 0.029 |
| Uric NNN ≥1.354 ng/dL | 0.798 (0.459, 1.387) | 0.423 |
| Likelihood ratio test | 0.017 |
*95% CI: (1.201, 1.507); Adjusted for age, sex, race, body mass index, alcohol intake, hypertension, diabetes mellitus, total cholesterol, triglycerides, serum uric acid, serum total calcium, serum phosphorus, serum creatinine, and total 25-hydroxyvitamin D. AAC, abdominal aortic calcification. NHANES, National Health and Nutrition Examination Survey. NNN, N′-nitrosonornicotine. CI, confidence interval.
Stratified and interactive analyses were performed to determine whether the association between uric NNN and severe AAC was stable among subgroups. In general, the association in the stratified analysis was consistent with that in the multivariate logistic regression analysis (Figure 2). The data indicated that age played an interactive role in the relationship between uric NNN and severe AAC (P = 0.033). In participants younger than 65 years, the association between uric NNN and severe AAC was stronger in the high uric NNN group (OR 3.66 [95% CI: 2.02–6.64, P < 0.001]) than the low uric NNN group. No significant association was observed in participants older than 65 years (OR, 1.51 [95% CI: 0.83–2.75, P = 0.176]). None of the other variables, including sex (female and male), BMI (<25 kg/m2 and ≥25 kg/m2), and race (Hispanic and non-Hispanic), significantly influenced the relationship between uric NNN and severe AAC (all P for interaction >0.05) (Figure 2).
Discussion
The present study investigated the dose–response relationship between uric NNN and severe AAC in noninstitutionalized US civilians. In general, uric NNN levels were positively associated with the risk of severe AAC. This study found the first evidence of a threshold effect of uric NNN on severe AAC, with a breakpoint of 1.354 ng/dL (95% CI: 1.201–1.507). Multiple potential covariates, including demographics, lifestyle, CVD risk factors, and laboratory measurements, were adjusted for in the analysis.
CS is a serious addiction and a leading modifiable risk factor for CVD [1, 13, 33, 34]. The underlying mechanisms include primarily endothelial dysfunction, oxidative stress, platelet activation, sympathetic activation, and inflammation [3, 7]. The biomarkers involved in these pathophysiological processes cannot accurately reflect cigarette consumption, owing to their lack of specificity. To precisely assess exposure to CS, particularly in passive smokers, and the condition of CS cessation, a reliable biomarker must be identified. The carcinogenic activities of nitrosamines have been well established over the past few decades [11]. TSNAs are among the specific compounds known to be present in tobacco smoke [14, 15]. In contrast to the well-documented carcinogenic activities [35], the association between TSNAs and CVD has rarely been discussed. To our knowledge, this is the first study to systematically examine the dose–response relationship between TSNAs and CVD. We focused on the most widely studied TSNA, NNN [12–15], and considered AAC as a predictor of CVD mortality, as previously documented [24–28]. Notably, both NNN and AAC can be quantitatively assessed noninvasively in hospital settings [29]. In this cross-sectional study, 2713 noninstitutionalized US civilians were recruited from the NHANES 2013–2014 (Figure 1). The normal range of uric NNN is unknown, because the relationship between NNN and CVD had not been previously investigated. In the present study, uric NNN levels were categorized as low or high, on the basis of the median concentration (0.2 ng/dL). The overall AAC prevalence was 29.9%, and the prevalence of severe AAC was 8.7%, a value similar to that reported in other studies based on NHANES 2013–2014 [18, 23, 30] (Tables 1 and 2). Additionally, both prevalence rates were higher in the high-NNN group than the low-NNN group (Tables 1 and 2). Although the association between uric NNN and severe AAC was not statistically significant in the crude model, uric NNN levels were positively correlated with the risk of severe AAC in all adjusted models (Table 2). The relationship between uric NNN levels and severe AAC is shown in Figure 3. According to the characteristics of the smoothing curve shown in Figure 3 (P for nonlinear = 0.025), we assumed the existence of a threshold effect of uric NNN levels on severe AAC, with one breakpoint. Therefore, we developed a two-piecewise linear regression model as shown in Table 3. The risk of severe AAC was found to increase 1.515-fold for every 1 ng/dL increase in uric NNN when the concentration of uric NNN is less than 1.354 ng/dL (95% CI: 1.201–1.507) (Table 3 and Figure 3). Subgroup analysis revealed that the association between uric NNN levels and the risk of severe AAC was stable between layers, except for age and race (Figure 2). A reasonable explanation for this finding may be the small sample size of the high-NNN group in each layer (Figure 2).
The mechanisms underlying the roles of TSNAs in the development of CVD have rarely been documented. Tithof et al. have suggested that 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a type of TSNA, induces endothelial cell apoptosis through beta 1- and beta 2-adrenergic receptor-mediated release of arachidonic acid [36]. In contrast, other studies have shown that NNK promotes angiogenesis in tumorigenesis [37, 38]. Penn and Snyder have indicated that the inhalation of butadiene (a cigarette smoke component), other than NNK, accelerates arteriosclerosis in vivo [39]. Further studies are required to identify the mechanisms through which TSNAs affect the occurrence and development of CVD.
Limitations
This study has several limitations. First, the causal relationship between uric NNN and severe AAC could not be assessed, because of the cross-sectional nature of this study. Second, the uric NNN tests were conducted with a single measurement, which might have underestimated the strength of the associations if a regression dilution bias existed. Third, the subjective description of several covariates in the database from NHANES 2013–2014, such as alcohol intake, might have influenced the results. Fourth, as a potential biomarker, the excretion and uptake of NNN may be affected by other factors, such as alcohol consumption. Finally, AAC was detected only in people 40 years of age or older, thus potentially influencing external validity. These limitations should be addressed in future studies.
Future Directions
In the future, more studies are needed to verify the association between TSNAs and CVDs. The mechanism through which TSNAs influence the development of CVDs must be determined, and cohort and interventional studies are needed to examine whether NNN might affect CVD prognosis. We believe that uric NNN may be a potentially reliable biomarker for evaluating the contribution of CS to CVD development, as well as the benefits of CS cessation in both active and passive smokers.
Conclusion
In a sample of noninstitutionalized US civilians, uric NNN levels were positively associated with the risk of severe AAC when the concentration of uric NNN was less than 1.354 ng/dL. This study demonstrated a previously unreported dose–response relationship between uric NNN and severe AAC, and a threshold effect of uric NNN levels on severe AAC. The relationships in the subgroup analysis were consistent with those in the multivariate logistic regression analysis.


