Introduction
Myocardial infarction (MI), a common manifestation of cardiovascular disease, has become the leading cause of death in humans [1]. During the pathological process of MI, the blood flow from coronary arteries to the heart is fractionally or completely occluded, thus resulting in irreversible apoptosis and necrosis of myocardial cells. Because terminally differentiated cardiomyocytes (CMs) cannot effectively regenerate, the damage to myocardial cells activates various adverse remodeling mechanisms, including arrhythmias [2], cardiac hypertrophy [3], and fibrotic scar tissue formation [4]. This adverse remodeling not only decreases electrical signal transmission and contractile activity, but also prevents the heart from functioning properly, and eventually results in heart failure (HF) [5]. Although coronary intervention and pharmacological thrombolysis have been developed to reconstruct coronary blood flow after MI, the irreversible damage to CMs limits the therapeutic effects [6]. Hence, innovative approaches are required to improve the growth and function of CMs, and prolong the stability of cardiac function.
Biomaterial-based scaffolds – artificial scaffolds that simulate the biochemical and physical features of the native extracellular matrix (ECM) and consequently improve cell-matrix interactions – are currently considered promising alternatives to prevent scarring and to promote myocardium remodeling after MI. These scaffolds, including microneedle patches [7], porous substrates [8], and hydrogels [9], not only provide mechanical support to infarcted areas, but also support cellular growth and function for myocardium repair. Among them, hydrogels have promising potential for in situ regeneration and repair of infarcted myocardium, because they provide a biomimetic, soft, and moist microenvironment [10]. With increasing knowledge regarding variations in the myocardial ECM after MI, intelligent and functional hydrogels have been synthesized, including immunomodulatory hydrogels to regulate inflammatory responses [11], conductive hydrogels to improve electrical coupling [12], and proangiogenic hydrogels to accelerate angiogenesis [13]. These hydrogels can be classified into injectable hydrogels and cardiac patch-based hydrogels [14]. Injectable hydrogels have gained substantial attention because of their injectable and in situ gelling features, and their superiority to cardiac patches in terms of their simple use, minimally invasive administration, and diminished risk of infection. This revolutionary approach offers a promising treatment option for repairing infarcted myocardium [15, 16]. To improve therapeutic effects, numerous injectable hydrogels have been developed to deliver therapeutic agents [17] and cells [18] locally to areas of infarcted myocardium. Although the effectiveness of these injectable hydrogels in MI therapies has been extensively demonstrated in small animal models, adequate investigations using large animals (such as porcine models) to establish a proof of concept supporting preclinical studies of MI treatment are lacking. Large animals with similar cardiac to body mass ratios and hemodynamics to those in humans can provide valuable spatial and temporal insights. In this context, understanding the interplay between the physicochemical properties of injectable hydrogels and infarcted myocardium repair is fundamental for advancing engineered substrates to actively manipulate myocardial cell behaviors, guide infarcted myocardium repair, and promote clinical applications. Therefore, a comprehensive overview of currently developed injectable hydrogels with a particular focus on their application for MI therapy is necessary.
Considering the many keywords associated with injectable hydrogels and MI (Figure 1), this review summarizes developed injectable functional hydrogels targeting various pathological issues in infarct areas for MI treatment. First, we discuss the pathophysiological mechanism of MI and key aspects of material design in MI therapy. Subsequently, we describe the development of injectable functional hydrogels and their action in targeting MI pathology, including reversion of the adverse microenvironment, restoration of electrical signal conduction, and/or promotion of angiogenesis, with an emphasis on the mechanisms of hydrogels in each category. Finally, clinical translation limitations and future perspectives of injectable hydrogels in MI treatment are summarized.
Material Designs for Repair of Infarcted Myocardium
Pathophysiological Mechanism of MI
Myocardial infarction occurs in severe heart diseases, including hypertension, heart valve disease, and coronary artery stenosis or obstruction [19–21]. Because of a failure in myocardium self-repair and insufficient regenerative capability, MI ultimately develops into HF, along with neurohumoral hyperactivity, an excessive inflammatory response, and chronic cardiac remodeling [22]. To better understand adaptive compensation after MI, this section focuses on the pathophysiological mechanism mediated by diminished myocardial systolic function and pathological processes in the chronic repair phase in infarcted myocardium.
MI is a typical manifestation of diminished myocardial systolic function induced by a decrease in the number of CMs [23], myocardial energy metabolism disorder [24], and myocardial excitation-contraction coupling disorder [25] (Figure 2A). The decrease in myocardial cells consequently decreases myocardial contractility [26, 27]. Myocardial energy metabolism disorders alter the structure and function of myocardial mitochondria, decrease myocardial energy synthesis, and increase local lactate production, thus further damaging the myocardium [28–31]. In myocardial excitation-contraction coupling disorder, a decrease in the content or activity of sarcoplasmic reticulum calcium-releasing proteins and Ca2+-ATPase leads to inhibition of myocardial contractility [32–35]. To preserve the integrity of the left ventricle (LV) after MI, ventricular remodeling occurs as a compensatory adaptive response through three overlapping stages: acute inflammation, proliferative repair, and mature remodeling (Figure 2B) [36, 37]. During the inflammatory phase, CM injury and death trigger various harmful outcomes in the first few days, including a decrease in pH, infiltration of neutrophils, and ECM degradation [37, 38]. Subsequently, a transition to the proliferative and repair phase occurs, and is accompanied by the suppression and regression of inflammation [39]. Resident fibroblasts subsequently migrate into infarcted regions and deposit collagen, thus resulting in scar formation to compensate for the loss of important mechanical support [40]. Finally, chronic remodeling of the damaged LV occurs over several months, and is characterized by ECM crosslinking and quiescence of myofibroblasts [37].
Basis the for Design of Biomaterials for MI Treatment
Biomaterial-based myocardium repair, a subset of tissue engineering, relies primarily on the (bio)physicochemical features of biomaterials to guide cell recruitment and growth, and then uses the recruited cells as building blocks to remodel damaged tissues [41–43]. When they adhere to biomaterials, cells can sense the physical cues (e.g., stiffness, surface patterns, and dimensionality) of substrates, acting in concert with other physicochemical and biochemical properties, through cell membrane-bound receptors [44–48]. The sensed extracellular signals activate a cascade of intracellular signaling that induces cellular responses involving cell migration, proliferation, and differentiation [49–51]. Cells in turn exert forces on the biomaterials and remodel their composition and structure through material degradation and ECM secretion. Biomaterials achieve their function for MI treatment primarily through two aspects: mechanical support and improvement in myocardial remodeling.
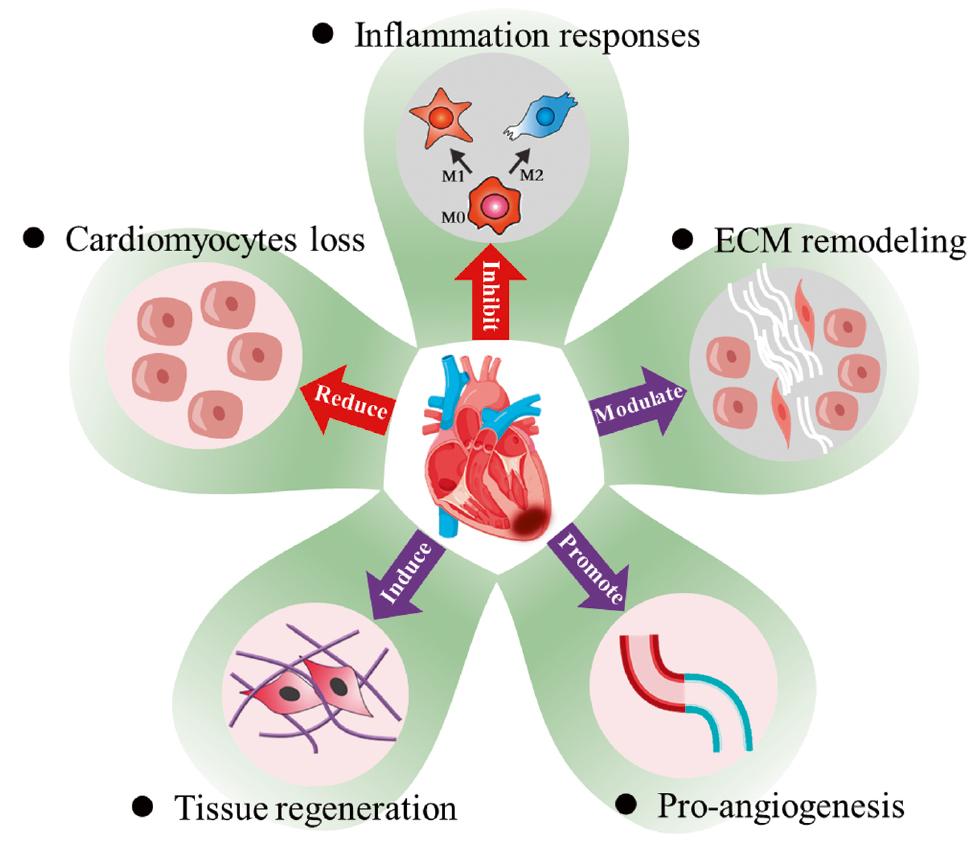
Because ventricular dilation and thinning of the LV wall contribute to elevated LV wall tension and myocardial oxygen consumption [52], the application of biomaterials provides temporary mechanical support to bear larger loads, thereby increasing ventricular wall thickness, decreasing ventricle stress, and improving myocardial remodeling [27, 53]. However, the increased local inflammation in infarcted region also tends to cause infarcted area dilation and wall thinning through upregulation of matrix metalloproteinases (MMPs) and disorders of the ECM remodeling [54]. In this context, to prevent further deformation and rupture of myocardial tissue, cells increase collagen deposition, thus leading to myocardial fibrosis. Therefore, biomaterials must be developed to attenuate local inflammation by enhancing reactive oxygen species (ROS) scavenging and inhibiting oxidative stress damage; preventing cell apoptosis through ECM replacement; and inhibiting scar hyperplasia via promoting fibrotic healing in infarcted areas and inhibiting reactive fibrosis in remote areas. Furthermore, by considering the series of biological changes in myocardial tissue, five processes can be targeted as a fundamental design basis for biomaterials intended for MI treatment (Figure 3): I) application of various apoptosis-inhibiting reagents or signals to decrease CM loss in infarcted myocardium; II) regulation of pro/anti-inflammatory cytokines/chemokines to inhibit immune cell infiltration and induce efficient tissue remodeling; III) persistent inhibition of myofibroblast activation to positively modulate ECM remodeling and fibrosis; VI) improvement in angiogenesis to restore the blood supply; V) promotion of myocardium self-repair via inducing CM cell cycle re-entry or promoting the migration of stem cells in infarcted myocardium.
Development of Injectable Hydrogels for MI Treatment
Given the constant contraction and relaxation of the heart, injectable hydrogels have received substantial attention as functional scaffolds for MI treatment, because of their minimally invasive administration through injection and their ability to provide mechanical support to infarcted areas, thereby limiting dilated ventricular size, improving ventricular shape, and thickening ventricular walls [55]. In initial investigations, injectable hydrogels were constructed primarily from natural or synthetic biomaterials alone. These hydrogels offer the potential to provide biochemical cues and modify the local tissue environment, and consequently prevent adverse left ventricular remodeling and decrease CM death. To establish clinical relevance, large animal studies have been conducted and demonstrated the efficacy of these injectable hydrogels in MI therapies. For instance, Mukherjee et al. have developed an injectable hydrogel called Fib-Alg by combining alginate with fibrin [56]. After implantation in a porcine MI model for 28 days, the Fib-Alg group and the healthy control group showed no statistically significant differences in functional echocardiographic measurements and had similar capillary density. Subsequent studies have confirmed the crucial roles of bioactivity and cellular interaction in influencing cardiac function [6, 57]. Consequently, injectable hydrogels with delivery systems for biologic agents were further developed and have shown remarkable efficacy in promoting cell growth and accelerating myocardial repair by limiting the diffusion of functional molecules, thus prolonging their local retention rate [58]. Various types of functional injectable hydrogels have been developed to overcome the limitations associated with current strategies for MI therapy (Table 1), including injectable conductive hydrogels [12, 60, 71], injectable stimulus-responsive hydrogels [66, 72, 73], drug delivery system-based injectable hydrogels [67, 74], and injectable self-healing hydrogels [69, 75] (Figure 4).
Development of Injectable Hydrogels for MI Treatment.
| Type of hydrogel | Merits | Materials | Preparation methods | Animal | Time | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Injectable conductive hydrogels | Restore normal electrical impulse propagation and resynchronize contractions | rGO-loaded GelMA/ODEX hydrogel | Schiff-base reaction and photo-crosslinking | Rat | 4 w | Enhanced expression of cardiac troponin I and Connexin 43, and decreased caspase-3 expression; improved ejection fraction | [59] |
| OXG/gelatin-PPy hydrogel | Schiff-base reaction | Rat | 4 w | Decreased electrical resistivity; decreased infarct size; increased vessel density; decreased inflammatory response | [60] | ||
| GO-loaded PEGDA700-Melamine/thiol-modified HA hydrogel | π–π conjugation and “thiol-ene” click reaction | Rat | 4 w | Increased α-smooth muscle actin and connexin 43 expression; increased ejection fraction; decreased fibrosis area and increased vessel density | [61] | ||
| DNA-eNOs-loaded TA-PEG/HA-SH hydrogel | Michael-type addition reaction | Rat | 4 w | Increased ejection fraction; shortened QRS interval; decreased fibrosis area; increased vessel density | [9] | ||
| TEMPOL and T59 peptide-loaded PPy/self-assembled peptides hydrogel | Self-assembling | Rat | 4 w | Decreased apoptosis; accelerated gap junction formation; increased ejection fraction; decreased fibrosis area | [12] | ||
| Injectable stimulus-responsive hydrogels | Respond to specific pathological issues in the infarct area for MI treatment | Poly(NIPAAm-co-VP-co-MAPLA-co-MATEMPO) ROS-responsive hydrogel | Free radical polymerization | Rat | 8 w | Decreased infarction/reperfusion injury; preserved left ventricle geometry | [62] |
| PAMB-G-TK/4-arm-PEG-SG ROS-responsive hydrogel | Michael-type addition reaction | Rat | 4 w | Scavenged excess ROS; improved mitochondrial dysfunction; promoted angiogenesis | [63] | ||
| Phenylboronic acid-grafted carboxymethyl cellulose ROS-responsive hydrogel loaded with PLGA@Cur NPs and rhCol III | Boronic ester bond | Rat | 4 w | Elevated expression of α-actinin and Connexin 43; decreased ROS and apoptosis; inhibited inflammatory response; accelerated angiogenesis | [64] | ||
| OSM-loaded poly (chitosan-co-citric acid-co-N-isopropyl acrylamide) dual-responsive hydrogel | Facile reversible addition-fragmentation chain transfer polymerization | Rat | 4 w | Accelerated angiogenesis and CM proliferation; inhibited myocardial fibrosis | [53] | ||
| UPy-coupled PEG pH-responsive hydrogel | Hydrogen bonding induced by alkyl-urea spacers and UPy | Pig | 4 w | Decreased collagen content | [65] | ||
| siMMP2-loaded HA MMP2-responsive hydrogel | Dynamic hydrazone bonds | Rat | 4 w | Improved myocardial thickness; increased ejection fraction, stroke volume, and cardiac output | [52] | ||
| hiPS-CMs and MMP2 peptide-loaded gold nanoparticle-hyaluronic acid MMP2-responsive hydrogel | Hyaluronic acid backbone crosslinked to MMP-2 peptide motif | Mouse | 4 w | Decreased fibrosis; improved conduction dysfunction of infarcted LV myocardium; promoted angiogenesis | [66] | ||
| Drug delivery system-based injectable hydrogels | Achieve optimal delivery of active drugs to the target site, and minimize patient risk | VEGF-loaded self-assembling peptide nanofiber hydrogel | Self-assembling | Rat and pig | 4 w | Improved angiogenesis and cardiac performance; recruited endogenous myofibroblasts | [57] |
| MicroRNA-21-5p-loaded MSN/PEGCHO/α-CD hydrogel | Hydrophobic and Schiff interactions | Pig | 4 w | Inhibited polarization of M1 macrophages; promoted local neovascularization | [67] | ||
| PEGDA-PBA/HA-SH/AST NP/GNR hydrogel | Hydrophobic self-assembling | Rat | 4 w | Inhibited left ventricular remodeling and myocardial dysfunction; upregulated infarct margin angiogenesis; decreased cell apoptosis | [26] | ||
| HA hydrogel modified with adamantane and thiols, or both β-cyclodextrin and methacrylates | Guest–host crosslinking and dual-crosslinking | Sheep | 8 w | Improved ejection fraction; increased infarct thickness | [68] | ||
| MSN-loaded puerarin-chitosan hydrogel | Self-assembling | None | None | Promoted angiogenesis; inhibited inflammatory response | [17] | ||
| Injectable self-healing hydrogels | Maintain the initial structure and functionality of hydrogels | SaB-PDA/pre-EMH hydrogel | Self-assembling | Rat | 4 w | Inhibited ventricular remodeling; promoted angiogenesis | [69] |
| Gelatin-UPy-Fe hydrogel | Strong tetrahydrogen bond | None | None | Increased drug release time of hydrogel | [70] |
Abbreviations: AST NPs: Astragaloside IV nanoparticles; EMH: elastin-mimic peptide hydrogel; eNOs: endothelial nitric oxide synthase; HA: hyaluronic acid; HA-SH: thiolated hyaluronic acid; hiPS: human induced pluripotent stem cells; hiPS-CMs: hiPS-derived cardiomyocytes; GelMA: gelatin methacryloyl; GNR: gold nanorod; GO: graphene oxide; MAPLA: methacrylate-polylactide; MATEMPO: methacrylate-TEMPO; MMP: matrix metalloproteinase; MSN: mesoporous silica nanoparticle; NIPAAm: N-isopropylacrylamide; ODEX: oxidized dextran; OSM: oncostatin M; OXG: oxidized xanthan gum; PAMB-G-TK: poly-3-amino-4-methoxybenzoic acid with TK-NH2-modified gelatin; PDA: polydopamine; PEG: poly(ethylene glycol); PLGA@Cur NPs: curcumin-loaded PLGA nanoparticles; PEGCHO: aldehyde-capped polyethylene glycol; PEGDA-PBA: polyethylene glycol diacrylate/4-vinyl phenylboronic acid; PPy: polypyrrole; rGO: reduced graphene oxide; rhCol III: recombinant humanized collagen type III; SaB: salvianolic acid B; siMMP2: siRNA against MMP2; TA-PEG: tetraaniline-polyethylene glycol diacrylate; TEMPO: 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl; TEMPOL: 4-hydroxy-TEMPO; UPy: ureido-pyrimid-inone; VP: vinylpyrrolidone; 4-arm-PEG-SG: 4-arm-PEGsuccinimidyl glutarate ester.
Injectable Conductive Hydrogels
The native myocardium is an electrically active tissue in which excitatory signals generated by pacemaker cells in the sinoatrial node propagate to the atrioventricular node and initiate atrial contraction [76]. The electrical excitement subsequently spreads through the atrioventricular bundle and Purkinje fibers, thus resulting in the depolarization of CMs and subsequent ventricular contraction [69]. However, CMs are generally replaced by non-contractible scar tissue in infarcted myocardium, thereby impeding electrical signal conduction in the myocardium, and leading to contractile asynchrony and arrhythmia [77]. To restore normal electrical impulse propagation and resynchronize contractions in infarcted myocardium, conductive materials have garnered substantial interest as “bridges” between the scarred and normal heart muscle, because of their semiconductor-like conductivity and ease of processing [77]. These materials markedly promote the maturation of cells responsive to electrical stimulation and consequently enhance intercellular communication [78]. In addition, injectable conductive hydrogels have shown notable efficacy in restoring interrupted electrical signal transmission in infarcted areas, thus ultimately resulting in synchronized contraction of the entire heart [79, 80].
Metallic nanomaterials (e.g., gold nanoparticles [81]), carbon-based materials (e.g., carbon nanotubes [82] and graphene [59]), and conductive polymers (e.g., polypyrrole [12], polyaniline [9], and polythiophene [83]) have frequently been used as conductive biomaterials in MI treatment. To develop injectable conductive hydrogels, these conductive materials are physically doped into hydrophilic injectable hydrogel matrices. However, the hydrophobic nature of these conductive materials often leads to their aggregation, thus resulting in rough and uneven clusters that transmit discontinuous electrical signals [84, 85]. This limitation severely limits the efficacy of injectable conductive hydrogels for MI treatment. To address this issue, enhancing the dispersion of conductive materials within injectable hydrogels has been considered an effective strategy to create a uniform conductive system. In this context, dopamine has been used as a reducing agent to convert graphene oxide (GO) into reduced graphene oxide (rGO), thereby enhancing the dispersion of GO in composite hydrogel matrices composed of gelatin methacrylate and oxidized dextran. This approach effectively accelerates the formation of a hydrogel system with good conductivity and uniformity [59]. In addition, modifying conductive materials with hydrophilic molecules is an effective strategy to improve their dispersibility within the injectable hydrogels. For instance, grafting gelatin molecules onto polypyrrole significantly enhances the water solubility of the conductive component, and enables more uniform incorporation into oxidized xanthan gum (OXG), as well as the formation of a uniform conductive system through Schiff-base reactions between OXG and gelatin [86]. These injectable hydrogels with improved conductivity have been reported to achieve significant decreases in infarct area and arrhythmia [60].
The conductivity of injectable hydrogels can also be enhanced through other approaches associated with the amount of conductive material, p-p conjugation, and the pacing threshold in the infarct area. For example, a high proportion of conductive material generally contributes to the superior conductivity of injectable hydrogels [87]. Because p-p conjugation enables long-range electron transport [88, 89], the introduction of p-p conjugation significantly enhances the conductivity of injectable hydrogels and facilitates the direct reconstruction of cardiac function [61]. Lowering the pacing threshold in the infarct area effectively decreases the electrical signals required for the normal beating, thus achieving indirect enhancement of electrical conductivity. This method is often used to support the administration of cardiac pacemakers and to decrease the threshold voltage of the pacemaker-stimulated myocardium [90]. Nevertheless, the ideal conductivity of hydrogel required for infarcted myocardium repair should be comparable to that of normal myocardium.
Injectable Stimulus-Responsive Hydrogels
After MI, various changes occur in the myocardial microenvironment, including fluctuations in the levels of MMPs, ROS, and local pH value. To target these pathological issues, researchers have developed various injectable stimulus-responsive hydrogels (e.g., MMP-responsive hydrogels [52], ROS-scavenging hydrogels [91], and temperature- or pH-responsive hydrogels [72, 73]) for use in myocardium repair processes.
MMPs, members of the zinc endopeptidase family that cleave ECM components, are considered key drivers of ECM remodeling in the myocardium [92]. The overexpression of MMPs in infarcted myocardium promotes ECM degradation and diminishes the mechanical properties of the ventricular wall, thereby resulting in progressive thinning and global dilatation of infarcted areas. Consequently, MMP levels have been used as a marker of HF [93]. For targeted inhibition of MMP expression and activity, the design of injectable hydrogels capable of regulating MMP levels has become a commonly used strategy for MI treatment. For example, Brendan et al. have reported the development of a polysaccharide-based hydrogel that can be injected locally into tissues and subsequently releases a recombinant tissue inhibitor of MMPs (rTIMP-3) in response to MMP activity [94]. This MMP-responsive hydrogel has been found to effectively inhibit adverse left ventricular remodeling in a porcine model.
Because of the abundant mitochondria in CMs, significant accumulation of ROS also plays a crucial role in impaired mitochondrial function and ATP metabolism after MI [95]. ROS directly attack DNA, proteins, and cell membranes; disrupt cell communication and signal transduction; and even cause cell death in myocardial and vascular cells outside infarcted areas, thereby exacerbating heart damage and increasing infarct size. Moreover, ROS stimulate the production of proinflammatory cytokines through various pathways, thus leading to a vicious cycle of ROS production and inflammation. Consequently, therapeutic interventions and ROS-responsive hydrogels have been developed to target ROS (e.g., superoxide anions, H2O2, and hydroxyl radicals) in the myocardial microenvironment to suppress oxidative stress injury [96]. For example, Wagner et al. have fabricated a ROS-sensitive hydrogel by incorporating 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) groups as ROS-scavenging pendants into a poly-N-isopropylacrylamide (NIPAAm)-based hydrogel. This ROS-responsive hydrogel effectively scavenges ROS radicals under oxidative stress and preserves the structure of the LV [62]. Furthermore, ROS-responsive hydrogels have been developed for localized delivery of liposome-containing drugs, thus enabling scavenging of excess ROS, ameliorating mitochondrial dysfunction in damaged CMs, and improving MI therapy [63].
Polymeric backbones with physicochemical structures that enable rearrangement in response to intrinsic (e.g., pH) and extrinsic stimuli (e.g., light) have also been developed into injectable stimulus-responsive hydrogels. These hydrogels are commonly designed as controlled drug release systems to optimize post-ischemic environments, and to promote CM function for the positive remodeling and repair of infarcted myocardium. For example, temperature-responsive hydrogels undergo a reversible transition from a soluble liquid to an insoluble gel phase at a lower critical solution temperature [97–99]. Above the critical temperature, they transition from a hydrophobic to a hydrophilic phase; consequently, the components can be injected into the liquid phase via a catheter and solidify into a gel under physiological conditions (37 °C) [73]. Through targeted thermo-responsive hydrogel therapy in conjunction with pro-angiogenic drugs, these injectable thermoresponsive hydrogels have been demonstrated to ameliorate cardiovascular remodeling and accelerate cardiac regeneration [53]. In addition, these temperature-responsive injectable hydrogels can be applied as cell delivery systems that allow cells to remain at the injection site for longer periods and recover more quickly from ischemia [73]. Given the low pH in infarcted myocardium compared with healthy cardiac tissue, pH can also be used as a targeted delivery stimulus to develop pH-responsive hydrogels [100]. As demonstrated by Bastings et al., pH-switchable supramolecular hydrogels not only exhibit self-healing properties but also can deliver growth factors to infarcted myocardium, thus decreasing infarct scars and improving cardiac function in MI pigs [65].
Drug Delivery System-Based Injectable Hydrogels
In the past, drugs were commonly administered through intramyocardial injections directly into the myocardium through either an epicardial method or a catheter technique [101]. Although epicardial injections are reliable, these invasive approaches have limitations in terms of accessing the septum and carry a risk of systemic embolization [91]. To achieve optimal delivery of active drugs to the target site while minimizing patient risk, the use of locally injectable hydrogels has emerged as a promising alternative with distinct advantages for drug delivery applications [75, 102–105]. These hydrogels allow drugs to be mixed with the precursor polymer solution before injection and subsequently become entrapped within the resulting hydrogel network [106–108]. Compared with systemic and oral administration, the use of these injectable hydrogels for topical drug delivery confers advantages in terms of sustained release and controlled biodistribution, thereby enabling gradual elution of drugs into neighboring tissues over prolonged periods of several days to months [109]. Moreover, these injectable hydrogels provide an intriguing avenue for mitigating MI progression though targeting adverse LV remodeling based on specific changes occurring in infarcted microenvironment via the sustained release of drugs.
Recently, a variety of drugs, including genes and proteins have been incorporated into injectable hydrogels to create drug delivery systems. For example, Lin et al. have developed an injectable hydrogel composed of self-assembling peptide nanofibers for the controlled release of recombinant human vascular endothelial growth factor (VEGF) [57]. Although promising results have been demonstrated in MI treatment in a porcine model, limitations exist with these hydrogels, particularly challenges in achieving sustained, on-demand, and pulsatile drug delivery that effectively responds to changes in the myocardial microenvironment [110, 111]. Because the release rate of drugs is influenced by external stimuli if the crosslinking reaction or procedure is reversible, various stimulus-responsive injectable hydrogels have been developed for drug delivery systems, which not only enable the slow release of drugs at target sites over long time periods but also deliver optimal drug concentration to infarction areas when needed [106]. For example, noncovalent injectable hydrogels commonly exhibit shear-thinning behavior that allows them to flow under applied stress and reorganize after the external stress is alleviated [75]. Such peptide-based self-assembled hydrogels, with tunable properties and biomimetic fibrous structure have emerged as promising drug delivery systems for MI treatment [112]. Nevertheless, challenges remain in controlling the quantity of injected material and avoiding the cell mortality induced by the administration of injectable hydrogels. Overall, new strategies coupled with accurate and controlled delivery of therapies should be explored to mitigate these shortcomings and achieve vacuum stabilization of the treated area for MI treatment.
Injectable Self-Healing Hydrogels
Because of the incessant beating of the heart, injectable hydrogels are prone to rapid destruction after injection, thus shortening their residence time in infarct areas. Although enhancing the mechanical strength of hydrogels can address this issue [113], this strategy may limit cell growth and hinder ingrowth of regenerative tissue within hydrogels. In this context, recent research has focused on self-healing hydrogels [43, 114–116], which maintain their initial structure and functionality through rapidly reaching an equilibrium between reversible dissociation and reorganization of functional groups within the hydrogel [117]. This reversible crosslink entails reversible chemical covalent bonds (e.g., imine bonds [118] or acylhydrazone bonds [119]) and physical non-covalent bonds (e.g., hydrogen bonds [120], ionic interactions [121], or p-p conjugation [122]).
The reversible imine bond, commonly known as a Schiff base, is a dynamic covalent bond formed by the reaction between an amine and an aldehyde group, which involves a dehydration reaction and does not produce any biotoxic byproducts [123]. Self-healing of hydrogels is enabled by the cleavage and reorganization of imine bonds within the hydrogel network under mild conditions [118]. For example, Basu et al. have developed a composite injectable hydrogel for the delivery of simvastatin based on DNA, oxidized alginate, and silicate-based nanoparticles [124]. These components enable the formation of imine bonds between the aldehyde groups of oxidized alginate and the amine groups present in DNA nucleotides, as well as electrostatic interactions between the phosphate groups of the DNA network and the charged silicate-based nanoparticles, thus forming hydrogels with self-healing and shear-thinning properties. In addition, an acylhydrazone bond can form through the reaction between an acyl hydrazine and aldehydes or ketones, thereby resulting in a covalent bond with pH-responsive properties [125]. In mildly acidic solutions (pH 4–7) consistent with the microenvironment of infarcted myocardium, the degradation of acylhydrazone bonds is particularly pronounced.
Hydrogen bonds are intermolecular interactions formed between hydrogen atoms and highly electronegative atoms. The self-healing ability involving hydrogen bonds is dependent on the extent of hydrogen bond formation and the chemical environment of the injectable hydrogel [120, 70]. Zhang et al. have developed an injectable self-healing hydrogel based on ionic interactions and hydrogen bonds [70], in which the ureidopyrimidinone (UPy) groups facilitate self-healing through UPy-UPy dimerization, and the addition of Fe3 further enhances the self-healing behavior through ion coordination with the carboxyl groups in gelatin. Recently, the formation of hydrogen bonds has been demonstrated to be associated with pH; the abundance of free hydroxyl, epoxy, carbonyl, and carboxyl groups; and electrostatic forces. For example, low pH conditions improve the self-healing ability of hydrogels, through increased hydrogen ion availability for enhanced hydrogen bonding [126]. Abundant free hydroxyl, epoxy, carbonyl, and carboxyl groups can engage in hydrogen bonding, thereby facilitating the formation of self-healing hydrogels [120]. Electrostatic forces contribute to the formation of self-healing hydrogels, because they allow for the dynamic and reversible fusion of polymer molecules within the three-dimensional network [127]. In addition, π-π conjugation refers to the dissociation of π electrons when two or more double bonds (or triple bonds) are linked by a single bond. For materials containing aromatic rings, such as graphene oxide, nucleic acids, and polydopamine (PDA), π-π conjugation can be used to design self-healing hydrogels [122], as demonstrated by Chen et al. [69]. Notably, π-π conjugation provides both conduction and self-healing functionalities and therefore is a promising approach for designing hydrogels for MI treatment.
Key Strategies for Injectable Hydrogels to Treat Myocardial infarction
After MI, various pathological issues occur in the infarct area, including blood vessel blockage, CM decrease, excessive inflammatory response, and myocardial fibrosis [13, 128, 129]. In normal tissues, adequate supply of oxygen allows myocardial cells to maintain normal vitality. However, after MI, the blockage of blood vessels decreases the supply of oxygen to the heart tissue, and consequently results in the apoptosis and necrosis of CMs, and impairment in the heart’s pumping function [13]. Meanwhile, the overactivation of inflammation increases ROS and disrupts cellular homeostasis, and the excessive formation of pathological fibrils hampers electrical signaling, thereby resulting in weakened myocardial contractile function, and promoting maladaptive healing and ventricular remodeling [37, 59]. To reverse this unfavorable milieu, several approaches, including restoring neovascularization, promoting CM proliferation, regulating inflammation, and decreasing myocardial fibrosis, have been used to restore cardiac function (Figure 5 and Table 2).
Key Strategies for Injectable Hydrogels to Treat Myocardial Infarction.
| Strategies | Features | Materials | Results | Ref. |
|---|---|---|---|---|
| Accelerating the restoration of myocardial vascularization | Inadequate blood and oxygen delivery; myocardial cell death | VEGF-loaded temperature-responsive hydrogel | Improved blood vessel density; inhibited cardiac remodeling; improved ventricular function | [130] |
| VEGF and ANG-1 delivery system-based hydrogel | Induced angiogenesis and blood vessel maturation; attenuated adverse myocardial remodeling | [27] | ||
| DNA-eNOs and ADSC loaded conductive hydrogel | Increased blood vessel density; improved cardiac function | [9] | ||
| VEGF and BMP9 delivery system-based hydrogel | Facilitated formation of blood vessels; inhibited myocardial fibrosis | [13] | ||
| AST NP and GNR delivery system-based hydrogel | Inhibited myocardial dysfunction; Upregulated infarct margin angiogenesis; decreased cell apoptosis | [26] | ||
| SaB loaded self-healing hydrogel | Inhibited ventricular remodeling; promoted angiogenesis | [69] | ||
| Myeloid-derived growth factor loaded delivery system-based hydrogel | Decreased scar formation and infarct size; increased wall thickness and neovascularization; improved heart function | [131] | ||
| SDF-1 and Ac-SDKP loaded delivery system-based hydrogel | Improved left ventricle function; increased angiogenesis; decreased infarct size | [132] | ||
| MSNs/microRNA-21-5p loaded delivery system-based hydrogel | Inhibited polarization of M1 macrophages; promoted local neovascularization | [67] | ||
| Promoting cardiomyocyte growth and differentiation | Recovering the functional constituents of the heart for periodic and autonomous contraction and relaxation | CM and VEGF delivery system-based hydrogel | Improved cell retention and integration, and decreased cell loss; alleviated ischemic microenvironment | [128] |
| Umbilical cord mesenchymal stem cells loaded conductive hydrogel | Enhanced expression of cardiac troponin I and Connexin 43; decreased caspase-3 expression; improved ejection fraction | [59] | ||
| Human mesenchymal stem cell loaded delivery system-based hydrogel | Decreased ejection fraction; improved vessel density; decreased infarction size | [18] | ||
| Histone deacetylase 7-derived-phosphorylated 7-amino-acid peptide loaded delivery system-based hydrogel | Restricted fibrosis of the LV wall; decreased infarct wall thinning; improved cardiac performance | [133] | ||
| microRNA loaded delivery system-based hydrogel | Promoted cardiomyocyte proliferation; improved ejection fraction | [129] | ||
| Decreasing myocardial fibrosis within infarcted myocardium | Decreasing excessive deposition of collagen and the fibro-inflammatory cascade reaction; inhibiting cardiac fibroblast activation | Yes-associated protein inhibitor (verteporfin) loaded delivery system-based hydrogel | Inhibited cardiac fibrosis; eliminated ROS | [134] |
| Oncostatin M loaded dual-responsive hydrogel | Accelerated angiogenesis; promoted proliferation of cardiomyocytes; inhibited myocardial fibrosis | [53] | ||
| Cur and rhCol III loaded ROS-responsive hydrogel | Elevated expression of α-actinin and Connexin 43; decreased ROS and apoptosis; inhibited inflammatory response; accelerated cell growth and angiogenesis | [64] | ||
| PEGDA700-Melamine and ADSC loaded conducive hydrogel | Increased α-smooth muscle actin and connexin 43 expression; increased ejection fraction; decreased fibrosis area and increased vessel density | [61] | ||
| Inhibiting excessive inflammatory responses | Inhibiting macrophage polarization into M1 phenotype’ decreasing ROS production | Puerarin loaded delivery system-based hydrogel | Decreased inflammatory response; stimulated angiogenesis | [17] |
| MSNs/microRNA-21-5p loaded delivery system-based hydrogel | Inhibited polarization of M1 macrophages; promoted local neovascularization | [67] | ||
| Ferulic acid loaded delivery system-based hydrogel | Inhibited ROS; improved cardiovascular function | [135] | ||
| Polydopamine-rosmarinic acid nanoparticle loaded dual-responsive hydrogel | Exerted anti-inflammatory effect; promoted synchronous contraction of cardiomyocytes; induced angiogenesis | [136] | ||
| Au@Pt nanoparticle loaded conducive hydrogel | Exerted anti-inflammatory and pro-angiogenic effects | [11] | ||
| HBPAK, CAT, and HA-MA loaded delivery system-based hydrogel | Inhibited apoptosis; increased blood vessels; decreased fibrosis; promoted cardiac function repair | [137] | ||
| Epigallocatechin-3-gallate and rhein-peptide delivery system-based hydrogel | Decreased formation of scarring; decreased ROS and inhibited inflammation; induced anti-apoptosis | [6] | ||
| Chitosan-vitamin C loaded thermo-responsive hydrogel | Improved survival of cardiomyocytes; decreased ROS level | [138] | ||
| Phenylboric acid and oxidized dextran loaded delivery system-based hydrogel | Decreased cell peroxidation and apoptosis; suppressed inflammation; preserved cardiac function and promoted LV vascularization | [139] |
Abbreviations: Ac-SDKP: angiogenic peptides; HBPAK: ROS-cleavable hyperbranched polymers; CAT: O2-generating catalase; HA-MA: methacrylate hyaluronic acid; SDF-1: stem cell homing factor.
Accelerating the Restoration of Myocardial Vascularization
Beyond its role in circulating blood throughout the body, the heart is a muscle that requires a consistent supply of oxygen and nutrients. If the blood vessels in the myocardium become blocked, the inadequate blood and oxygen delivery to a segment of the heart muscle is exacerbated, thus leading to tissue oxygen deprivation, known as cardiac ischemia. Prolonged oxygen deprivation contributes to myocardial cell death and ultimately results in MI or heart attack [140]. Therefore, reestablishing vascularization in infarcted areas is crucial for MI treatment. Neovascularization, which facilitates the provision of oxygen and nutrients to infarct areas, not only ameliorates the adverse microenvironment associated with MI and enhances the survival rate of transplanted cells, but also prevents further expansion of infarcted region [141, 142]. In this context, researchers have explored the incorporation of various vascular regulatory factors [143], plasmids [9], or drugs [17, 26] into injectable hydrogels to accelerate the restoration of myocardial vascularization.
VEGF is a commonly used proangiogenic factor that binds VEGF receptors on endothelial cells, and promotes the autophosphorylation and migration of endothelial cells (ECs) through activation of the Src pathway [144]. Given the short half-life of VEGF [13], Wu et al. have incorporated VEGF into an aliphatic polyester hydrogel to extend the release of the factor, thereby significantly improving high blood vessel density in infarcted myocardium [130]. Angiopoietin-1 (ANG-1) is another growth factor that promotes angiogenesis and has the unique advantage of inhibiting vascular leakage [145, 146]. Rufaihah et al. have used a polyethylene glycol-fibrinogen hydrogel to co-deliver VEGF and ANG-1, thus overcoming the limitations associated with single growth factor treatment for arteriogenesis [27]. In addition, platelet-rich fibrin contains platelet-derived growth factor, VEGF, and transforming growth factor (TFG) β-1, and therefore is highly effective in promoting angiogenesis [147, 148]. Basic fibroblast growth factor and myeloid-derived growth factor have also been applied to promote neovascularization and restore cardiac function [72, 131].
The recent successful completion of a phase 2 clinical trial on gene therapy for MI highlights the potential of gene therapy as a broad therapeutic approach for cardiovascular diseases [149, 150]. Given that nitric oxide plays key regulatory roles in endothelial cell growth, migration, and angiogenesis [151], Wang et al. have fabricated a conductive injectable hydrogel carrying lipid amine (Lipo)/plasmid DNA-eNOs (endothelial nitric oxide synthase) nanocomplexes and adipose-derived stem cells (ADSCs), in which the plasmid can be transferred into ADSCs and result in continuous nitric oxide production. In vivo experiments with this injectable hydrogel have demonstrated a significant increase in blood vessel density within the infarct area and improved cardiac function [9]. The advantage of this approach lies in the continuous release of regulatory factors through integration into host cell gene expression after DNA integration. However, gene therapy poses challenges in the difficulties in nucleic acid delivery into cells, and the cumbersome and expensive production process. Therefore, further exploration and research are required to overcome these limitations.
Promoting Cardiomyocyte Growth and Differentiation
CMs notably constitute the myocardium and serve as the functional constituents of the heart. Their proper functioning determines the heart’s inherent capability for periodic and autonomous contraction and relaxation. After maturation, these cells lack the ability to proliferate, thus rendering them permanent cells [152]. Consequently, incidents such as MI may cause irreversible decreases in CM number and consequently impair the ability of the heart to efficiently circulate blood throughout the body. In this context, researchers have proposed the development of injectable hydrogels that enhance the proliferation and regeneration of CMs in conjunction with stem cell therapy [18, 128], mRNA therapy [129, 153], or cytokine therapy [143, 154].
Induction of stem cell differentiation into CMs is considered a promising approach for MI treatment. Given their unlimited self-renewal capability and ability to generate highly differentiated daughter cells, stem cells, such as mesenchymal stem cells [18, 59], embryonic stem cells [155], induced pluripotent stem cells [156], and ADSCs [157], have been widely used in MI treatments [158]. These stem cells can differentiate into myocardial precursor cells and eventually mature into functional CMs [159]. The relevant key signaling pathways include Wnt [160], BMP [161], and FGF [162]. Differentiated CMs, replacing lost CMs, release paracrine signaling molecules and enhance the structural integrity and functional activity of the myocardium [163]. However, a challenge of low survival rates among implanted cells arises during hydrogel preparation. To address this issue, Liu et al. have incorporated fibrin and VEGF into alginate-based injectable hydrogels containing neonatal CMs, which have been found to effectively improve cell retention, integration; decrease cell loss; and alleviate the ischemic microenvironment in the infarct area [128]. Another strategy to improve cell survival is the microencapsulation of cells in small beads. For instance, Gal et al. have incorporated ECM from omental tissue and induced pluripotent stem cell-derived CMs, generated cell droplets by using a microfluidic system, and subsequently introduced them into hydrogels. This microencapsulation protects the cells by preserving their cellular functions and shielding them from the environment [164–166]. Nevertheless, an ideal injectable hydrogel that encapsulates cells should not only provide mechanical support for CM growth but also restore myocardial vascularization and electrical conductivity, because impaired electrical signal transfer and limited oxygen supply restrict cell survival [59, 164].
Drugs, including cytokines, are also used in therapeutic strategies for MI treatment. Stromal-derived factor-1 (SDF-1), functioning as a homing factor, exerts a chemotactic effect on mesenchymal stromal cells and ECs to ischemic regions through binding the receptor CXCR4, thereby stimulating cell growth, proliferation, and migration [167, 168]. Ac-SDKP (N-acetyl-seryl-aspartyl-lysyl-proline), a small tetrapeptide derived from thymosin β-4 via proline endopeptidase or prolyl oligopeptidase, promotes ECs proliferation and angiogenesis, thus potentially facilitating cell proliferation after MI [169, 170]. Researchers have incorporated SDF-1 and Ac-SDKP peptides into HA-based hydrogels to evaluate their regenerative potential in the context of myocardium healing [132]. In addition, a collagen hydrogel enriched with a peptide derived from HDAC7 has been shown to enhance the formation of new microvessels, the recruitment and differentiation of stem cell antigen-1 positive (Sca-1+) stem cells, and the growth of CMs. This approach effectively promotes the repair of infarcted myocardium [133].
Decreasing Myocardial Fibrosis within Infarcted Myocardium
Myocardial fibrosis is a multiphase reparative response following ischemic cell death during MI [171]. Under pathological conditions, cardiac fibroblasts undergo transformation into myofibroblasts [172, 173], thus promoting the synthesis and accumulation of collagen fibers in the ECM. Excessive fibrosis then results in the replacement of necrotic myocardial cells with fibrotic scars, in a process involving the several factors (e.g., TGF-β, interleukin-4, interleukin-10, interleukin-13, and angiotensin II) as well as the Hippo and STAT6 pathways [171]. This fibrotic response subsequently induces geometric, biomechanical, and biochemical changes in the uninjured ventricular wall; triggers a reactive remodeling process; and hinders the transmission of myocardial electrical activity. Although the initial reparative fibrosis is essential for preventing ventricular wall rupture, an exaggerated and reactive fibrotic response beyond the injured area can be detrimental, by progressively impairing cardiac function and ultimately leading to HF [174]. Furthermore, myofibroblasts electrically connect to CMs, alter the CM structural phenotype, and increase CM stiffness, thereby preventing the expansion of the infarct zone and ultimately resulting in HF [175].
MyoFbs, but not Fbs, readily form connections with CMs in direct co-culture. Connexin 43 expression in MyoFb is higher than that in Fb. When coupled to CMs, MyoFbs decrease the CM action potential duration and hyperpolarize the CM resting membrane potential, whereas uncoupling reverses these effects. In conclusion, MyoFbs, but not Fbs, alter the CM structural phenotype. MyoFbs, but not Fbs, are likely to electrically connect to CMs and thereby modulate the CM membrane potential.
Inhibition of fibrosis-associated cytokines and pathways is considered a viable approach to counteract myocardial fibrosis. For instance, the activation of the Hippo pathway has been closely associated with pathological fibrosis in heart disease. Yes-associated protein (YAP), a key factor in the Hippo signaling pathway, plays a critical role in fibrosis development post MI. In this context, Sun et al. have designed an injectable hydrogel based on thiol-modified poly(γ-glutamic acid) and incorporated a YAP inhibitor (verteporfin), which has demonstrated a significant decrease in excess collagen deposition in infarcted myocardium after the application of verteporfin [134]. In addition, oncostatin M (OSM), a bioactive factor secreted by macrophages, inhibits cardiac fibroblast activation by directly affecting TGF-β1 through extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation in the S-mad junction region. OSM also decreases myocardial collagen deposition through the activation of SIRT1 and the upregulation of SMAD7 [176]. Consequently, an injectable hydrogel composed of poly(chitosan-citric acid-N-isopropylacrylamide) loaded with OSM has been fabricated to decrease myocardial fibrosis. This hydrogel exhibits local release of OSM in response to specific pH values [53]. Furthermore, alternative strategies such as enhancing electrical transmission and scavenging ROS have also shown potential in inhibiting pathological cardiac fibrosis [64], thus providing valuable insights into integrating multiple strategies for MI treatment.
Inhibition of Excessive Inflammatory Responses
Oxidative stress is a major pathological mechanism in infarcted myocardium at the cardiovascular level. Intense oxidative stress leads to sustained damage to CMs through various pathways, thereby resulting in CM apoptosis, autophagy, and irreversible cardiac dysfunction [6, 177]. The injured cells subsequently release danger-associated molecular patterns, and activate Toll-like receptor 4 (TLR4) and nuclear factor kappa-B (NF-κB), thus ultimately triggering the recruitment of chemokines and pro-inflammatory cytokines [6]. The recruited chemokines and cytokines in turn mediate immune cell recruitment and promote the production of ROS through the expression of NADPH oxidase [137, 178]. Notably, deficiencies in ROS in humans result in severe recurrent bacterial infections, whereas elevated ROS formation leads to molecular damage and pathology due to excessive inflammation [179]. Therefore, controlling the expression of pro-inflammatory factors or specific ROS-mediated inflammation is a promising avenue for MI treatment.
M1 macrophages play a critical role in activating acute inflammatory reactions in infarcted areas [180]. They secrete substantial amounts of pro-inflammatory cytokines (e.g., interleukins, interferon-γ, and chemokines) and exhibit high expression of inducible nitric oxide synthase and reactive oxygen intermediates [181]. Therefore, inhibiting macrophage polarization into the M1 phenotype is an effective strategy to regulate inflammatory responses. For instance, puerarin, a traditional Chinese medicine known for its ability to inhibit macrophage M1 polarization, has been incorporated into a chitosan hydrogel combined with mesoporous silica nanoparticles to promote angiogenesis. Gene expression analysis has demonstrated that the addition of puerarin effectively decreases the expression of pro-inflammatory factors in cells [17]. Moreover, type I collagen hydrogel supplemented with firistine has been found to have anti-inflammatory properties by decreasing the number of pro-inflammatory macrophages [182].
The term ROS collectively refers to various derivatives of molecular oxygen that arise as byproducts of oxygen metabolism in aerobic organisms [183]. These byproducts include hydrogen peroxide (H2O2), hydroxyl radical (·OH), and superoxide anion (O2 −) [179, 184–186]. Alongside inhibiting M1 macrophage polarization, scavenging ROS in infarcted areas has emerged as an effective anti-inflammatory strategy for MI treatment. Recently, injectable hydrogels have been developed to scavenge ROS by incorporating antioxidants such as ferulic acid [139], curcumin [64], astragaloside IV [26], vitamin C [138], and TEMPOL [12]. For instance, the inclusion of the antioxidant ferulic acid in a chitosan-gelatin-based hydrogel effectively decreases oxidative stress through increasing catalase activity and inhibiting endogenous ROS production [135]. In addition, the utilization of boron ester bonds, known for their ROS-scavenging properties, has led to the development of ROS-responsive injectable hydrogels [95, 139]. For instance, in conditions of excessive inflammation, ROS can cleave the boron ester bond formed between the phenylboronic acid group and the catechol group in hydrogels, thus inducing the release of functional molecules and improving MI treatment [136].
Conclusions and Future Prospects
Injectable hydrogels have substantial promise for MI repair. In conventional approaches, direct delivery of cells or drugs into infarcted regions is hindered by blood flow, thus resulting in low retention rates and limited therapeutic efficacy. By acting as carriers for cells, drugs, and biomolecules, injectable hydrogels enhance localization within infarcted areas, and simultaneously offer superior mechanical properties. Several types of functional hydrogels have emerged, including conductive hydrogels, stimulus-responsive hydrogels, drug delivery system-based injectable hydrogels, and self-healing hydrogels. Conductive hydrogels can reinstate electrical conductivity in infarcted myocardium, thereby facilitating the restoration of systolic and diastolic rhythms, and synchronized cardiac contractions. Stimuli-responsive hydrogels are often designed in tandem with drug delivery systems to target infarcted tissue, maximize drug utilization, and increase tolerance to potential injection errors. In addition, self-healing hydrogels have the unique ability to repair fractures within themselves, thus enabling prolonged interaction with the heart and exhibiting “two-in-one” therapeutic properties. These advancements in injectable hydrogels have substantial potential for mitigating the effects of MI and aiding in the recovery of cardiac function.
Although notable progress has been made, the currently available injectable hydrogels fall short of meeting clinical requirements for MI repair. Therefore this field requires substantial advancement. For example, the regulatory mechanisms of injectable hydrogels in improving cardiac repair and regeneration involve enhancing vascularization in damaged tissue, promoting CM proliferation and regeneration, alleviating the inflammatory response, and inhibiting fibrosis. These mechanisms are interconnected, and addressing any one of them can improve the microenvironment of infarcted myocardium. Hence, the complexity of these molecular mechanisms necessitates the exploration of injectable multifunctional hydrogels that simultaneously inhibit fibrosis, decrease inflammation, promote angiogenesis, and facilitate CM growth for MI repair. Furthermore, although small animals, such as rats, mice, and rabbits, have been extensively used to assess the effectiveness of these injectable hydrogels in MI treatment, research on their applications in large animals and humans remains lacking. Adequate investigations will be crucial before these hydrogels can be considered for clinical implementation. Notably, traditional Chinese medicines have shown promise in treating MI through anti-inflammation and anti-fibrosis activity. Hence, incorporating these medicines into drug delivery-based injectable hydrogels may also offer a viable strategy for further development.
In summary, by alteration of their physical and chemical properties or incorporation of therapeutic molecules, injectable hydrogels can be endowed with diverse functionalities that expand their potential in the field of cardiac diseases. We believe that injectable hydrogels have substantial translational prospects for cardiac repair and regeneration. Therefore, further investigation of their clinical viability is warranted.