Introduction
Cardiovascular disease is one of the leading causes of death and disability worldwide. Although myocardial reperfusion treatments such as thrombolysis or percutaneous coronary intervention are commonly used for patients with myocardial infarction, they may not always effectively prevent myocardial injury [1, 2].
Stem cells play important roles in repairing and regenerating damaged tissue, mainly via the paracrine secretion of tissue-regenerative factors. Stem cells may be therapeutically effective because of exosomes. Various mesenchymal stem cells have been shown to release exosomes that increase vascular generation, decrease inflammation, and alleviate oxidative stress [3]. Stem cell-derived exosomes are important in repairing and regenerating damaged tissue, particularly in neurodegenerative disorders and heart disease [4]. Several animal and human studies have shown that exosomes inhibit myocardial apoptosis, decrease myocardial infarction, preserve left ventricular geometry, and increase cardiac function after myocardial ischemia-reperfusion injury [5]. Exosomes are small membrane-bound vesicles released by cells into the extracellular space. Their diameters can range from 30 to 150 nm, and they can contain various biomolecules, such as proteins, lipids, and RNAs including microRNAs (miRNAs) and messenger RNAs (mRNAs) [6, 7]. ADSCs also have critical functions in mediating tissue repair [8]. ADSCs secrete subcellular granules of ADSC-Exos, which are approximately 20–100 nm in diameter. Compared with ADSCs, ADSC-Exos have higher stability and lower antigenicity, and thus can confer long-term protection against tissue damage-related disorders. Although our previous study has demonstrated the protective effects of ADSC-Exos against cardiomyocyte injury, the mechanism underlying this protection remains unclear [9].
ADSC-Exos contain a diverse array of RNAs and proteins, including circRNAs. These endogenous, non-coding RNA molecules play critical roles in regulating gene expression and therefore may enable understanding of the mechanisms involved in the development of ADSC-Exo protective effects. circRNAs are formed through a process known as back-splicing, in which the 5′ end of a pre-mRNA upstream exon is non-collinearly spliced with the 3′ end of a downstream exon, thus forming a circular molecule. Compared with linearly structured miRNAs and mRNAs, circRNAs resist exonuclease-mediated degradation, because their structures lack a 5′ cap and 3′ tail [10, 11]. Hence, circRNAs are usually more stable than linear RNAs and therefore can exert prolonged biological effects. Mechanistically, circRNAs can act as ceRNAs that competitively influence the functions of miRNAs and their target genes in various pathological processes [12], including cardiovascular diseases. For example, the circRNA_MFACR exacerbates myocardial infarction via upregulation of MTP18 expression [13]. Similarly, circRNA_NCX1 mediates myocardial injury by suppressing the function of miR-133a-3p, thus increasing the expression of CDIP1 [14], whereas circRNA_000203 promotes cardiac hypertrophy by inhibiting binding of miR26b-5p and miR-140-3p to Gata4 [15]. However, the expression profiles and therapeutic potential of ADSC-Exo-derived circRNAs in cardiomyocyte injury remain elusive.
In this study, we aimed to screen differentially expressed circRNAs in ADSCs and ADSC-Exos to explore the effects and mechanisms of ADSC-Exos in cardiomyocyte injury. Our results may provide an experimental foundation for the development of new therapeutic interventions for cardiomyocyte injury.
Methods
Cell Culture and Exosome Isolation
Established methods were used for ADSC and ADSC-Exo cell culture [9]. The ADSCs, which were acquired from Cyagen Biosciences Inc. (cat. MUBMD-01001), were cultured in Dulbecco’s modified Eagle’s medium/F12 (1:1) medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum. The expression of mesenchymal stem cell markers, with antibodies including anti-human CD29 APC (1:1000; eBioscience) and FITC mouse anti-human CD44 (1:1000; BD Pharmingen), was confirmed through flow cytometry analysis, whereas the cells tested negative with anti-human CD34 FITC (1:1000; eBioscience). The extracted exosomes exhibited high purity, as determined with a MagCaptureTM Exosome Isolation Kit PS (Wako, cat. 293-77601). Morphological examination of the ADSC-Exos through electron micrography revealed the presence of grape-like nanoparticle exosomes.
RNA Extraction and circRNA Microarray Analysis
RNA samples from the ADSC and ADSC-Exo groups were first extracted with an RNeasy Total RNA Isolation Kit (Qiagen, Germany) according to the manufacturer’s instructions. The amount and purity of total RNA were evaluated with a NanoDrop ND-2000 spectrophotometer. The samples were then targeted with a One-Color RNA Spike-In Kit from Agilent Technologies. After hybridization of the targeted samples, slide scanning was performed with an Agilent Microarray Scanner. The raw data were normalized with the Quantile algorithm of the limma package in R. A fold change cutoff of 2 was applied in the analysis.
The limma package in R software was used to study the differential circRNAs. Fold change (linear) ≤ 0.5 or fold change (linear) ≥2 were defined as the thresholds for differential expression of RNAs. Moreover, the Fisher’s exact test was used to identify genes with significant differential expression based on Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses, with the clusterProfiler package in R software. The screening criteria were P < 0.05 and count ≥2.
Quantitative Real Time-PCR
QRT-PCR was performed to quantify the levels of three upregulated circRNAs (circ-0008302, circ-0010012, and circ-0007490) with the QuantStudio™ 6 Pro system (Thermo Fisher Scientific, USA). Extracted RNA was subjected to cDNA synthesis with a HiScript Reverse Transcriptase kit (Vazyme, China). QRT-PCR was conducted with SYBR Green Master Mix (Vazyme), according to the manufacturer’s instructions and the threshold method for quantitative analysis. With glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal reference, the corresponding ΔΔCt values in each group of cells were calculated, and quantitative analysis was performed according to the quantity of the target factor (2−ΔΔCt). All primers are listed in Table 1.
Primer Sequences Used for Detecting circRNAs.
| Name | Primer | Sequence |
|---|---|---|
| Mus GAPDH | Forward | 5′-ATGGGTGTGAACCACGAGA-3′ |
| Reverse | 5′-CAGGGATGATGTTCTGGGCA-3′ | |
| Mus circRNA-0007490 | Forward | 5′-GGAACCTATACTTGCGTTGTCA-3′ |
| Reverse | 5′-GAACCTCAATGGCCCCA-3′ | |
| Mus circRNA-0008302 | Forward | 5′-TCCTTGCATTCCCTATTTAGCAG-3′ |
| Reverse | 5′-CACTTTCTTCATAGCCAGACCATC-3′ | |
| Mus circRNA-0010012 | Forward | 5′-GAGGAAAGTCCCAAAGCAAA-3′ |
| Reverse | 5′-CACCACAGGACGCATTGAC-3′ |
Construction and Validation of circRNA siRNA Plasmids
The obtained ADSC-Exos and differentially expressed circRNAs in the exosomes were divided into three groups: circ-0008302 siRNA1, circ-0008302 siRNA2, and circ-0008302 siRNA3. The identified differentially expressed circRNAs were verified by qRT-PCR. All primers are listed in Table 2.
Primer Sequences of circ-0008302 siRNAs.
| Name | Primer | Sequence |
|---|---|---|
| Mus-circRNA-0008302-si1 | Forward | 5′-CAUUCCCUAUUUAGCAGUUTT-3′ |
| Reverse | 5′-AACUGCUAAAUAGGGAAUGTT-3′ | |
| Mus-circRNA-0008302-si2 | Forward | 5′-CCUAUUUAGCAGUUGAGCATT-3′ |
| Reverse | 5′-UGCUCAACUGCUAAAUAGGTT-3′ | |
| Mus-circRNA-0008302-si3 | Forward | 5′-CUAUUUAGCAGUUGAGCAUTT-3′ |
| Reverse | 5′-AUGCUCAACUGCUAAAUAGTT-3′ | |
| NC | Forward | 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Reverse | 5′-ACGUGACACGUUCGGAGAATT-3′ |
Cell Viability Analysis
Culture of M6200 mouse cardiomyocytes was performed according to previously published methods [9]. The M6200 cells were divided into four groups: a control group, H2O2 induced group, H2O2 + ADSC-Exo group, and H2O2 + ADSC-Exommu-circ-0008302 siRNA group with treatment with 200 μM H2O2 for 4 h. M6200 cells in logarithmic growth phase were seeded into 96-well plates with 100 μL Dulbecco’s modified Eagle’s medium per well. After the cells adhered to the plate, the original medium was removed. The cells were then treated with 10 μL of Cell Counting Kit-8 solution per well and incubated for 4 hours, according to the manufacturer’s instructions. The cell viability was then determined by measurement of the light absorbance at 450 nm with a microplate reader.
Flow Cytometry Analysis
Levels of apoptosis were measured with flow cytometry. In this analysis, M6200 cells were seeded into six-well plates and placed in a cell culture incubator with 5% carbon dioxide (CO2) at 37°C for 24 hours. After the cells had adhered to the plate, they were subjected to the experimental treatments for 24 hours. The cells were then centrifuged, washed, and harvested for flow cytometry analysis. CELL Quest software was used to analyze the apoptosis levels in each group.
Similarly, for intracellular reactive oxygen species (ROS) analysis, M6200 cells were plated into six-well plates and placed in a 5% CO2 cell incubator at 37°C for 24 hours. After cells had adhered and had been subjected to experimental treatments, they were digested with 0.25% trypsin without ethylenediaminetetraacetic acid and washed. A reactive oxygen species assay kit (Beyotime, China) was used according to the manufacturer’s instructions. The fluorescent DCFH-DA probe was incubated with cells for 20 min at 37°C at a concentration of 10 mM. The ROS levels in each cell group were detected with flow cytometry.
QRT-PCR and Western Blot Analysis
Subsequently, qRT-PCR was used to detect the miRNA levels. All primers are listed in Table 3. After the cells were treated, the culture medium was aspirated, and the cells were washed with cold PBS. After addition of 2 μL PMSF and 2 μL phosphatase inhibitor, the cells were lysed on ice for 30 min at 4°C and centrifuged at 12,000 rpm for 5 min. The supernatant was used for protein quantification. After SDS-PAGE separation and membrane transfer, blocking was performed at room temperature. Subsequently, the membranes were incubated with primary antibody (MsrA, Abcam, USA) and GAPDH primary antibody (Goodhere Biology, China) and incubated at 4°C overnight. Goat anti-rabbit IgG secondary antibody (Boster, China) was applied for 2 h before chemiluminescence was detected.
Statistical Analysis
The data obtained from the indicated experiments are expressed as mean ± SD and were analyzed in SPSS 17.0 software (SPSS Inc., IBM, Armonk, NY, USA). One-way analysis of variance was used to compare variables between the control and experimental groups. For all statistical tests, a P-value below 0.05 was considered statistically significant.
Results
Profiling of Differentially Expressed circRNAs
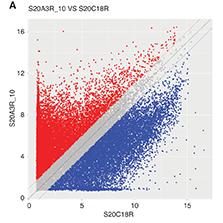
The circRNA expression profile comparison between ADSCs and ADSC-Exos revealed several highly upregulated circRNAs in the ADSC-Exos, including circ-0008302, circ-0002468, circ-0002157, circ-0008982, circ-0008455, circ-0010012, circ-0009075, and circ0007490 (Table 3). A comparison of the host genes and functions of these circRNAs, on the basis of the literature, indicated that circ-0008302 is highly expressed in the heart, and has critical functions in cell proliferation, differentiation, and survival [16, 17]; circ-0002157 (host gene Rnf217) is associated with poor prognosis in myeloma [18]; circ-0008982 (host gene Serpine2) promotes myocardial fibrosis [19, 20]; circ-0008455 (host gene Rgs7) is induced in the dentate gyrus after ischemia [21]; circ-0010012 (host gene Cacnb4) encodes a calcium channel subunit expressed in the heart and involved in myocardial contraction [22]; circ-0009075 (host gene Hdac4) is associated with myocardial exercise intensity and enhances HDAC4-NT levels [23]; and circ0007490 (host gene Dcc) is associated with central nervous system dysfunction caused by abnormal axon signal conduction [24]. On the basis of the above findings, we selected circ-0008302, circ-0010012, and circ-0007490 for further validation by qRT-PCR. The primer sequences are presented in Table 2. Compared with the control group, the ADSC-Exo group had significantly lower expression levels of circ-0007490 and circ-0010012, and significantly higher expression levels of circ-0008302. Therefore, we selected circ-0008302 for subsequent experiments (Figure 1).

The expression of circRNAs in ADSCs and ADSCs-Exo.
A. circRNA scatter plot: The red dots on the plot indicate the upregulated differential genes with a fold change greater than 2 (ADSCs-Exo versus control), while the blue dots represent the downregulated differential genes. B. The expression levels of the circRNAs in ADSCs and ADSCs-Exo were evaluated by qRT-PCR compared with the ADSCs group, circRNA expression of the control group was designed as 1. **P < 0.01.
Silencing of circ-0008302 Expression with siRNA
To investigate the gene silencing effect of circ-0008302, we transfected M6200 mouse cardiac myocytes with circ-0008302 siRNA. The expression of circ-0008302 did not differ between cells transfected with a negative control siRNA (siRNA-NC) and the control group. The primer sequences of circ-0008302 siRNAs and siRNA-NC are presented in Table 3. In cells transfected with circ-0008302 siRNA1, 2, and 3, the expression of circ-0008302 was significantly diminished. Because siRNA2 showed the most prominent effect, circ-0008302 siRNA2 was chosen for subsequent experiments (Figure 2).
Effects of ADSC-Exos and circ-0008302 in H2O2-Induced Cardiomyocyte Injury
The effects of ADSC-Exo and circ-0008302 on cell proliferation and apoptosis in H2O2-induced cardiomyocyte injury are illustrated in Figure 3. Treatment with H2O2 resulted in significantly lower viability of M6200 cardiac myocytes than control cells. However, this diminished viability was rescued by ADSC-Exos. The protective effect of ADSC-Exos was dependent on the presence of circ-0008302, because circ-0008302 knockdown abrogated the protective effect (Figure 3A). Compared with the control group, the H2O2 treatment group showed greater cardiomyocyte apoptosis, whereas ADSC-Exo treatment significantly decreased cell apoptosis. However, the anti-apoptotic role of ADSC-Exos was significantly abrogated by circ-0008302 knockdown (Figure 3B, C). The results indicated that ADSC-Exos may protect against ischemic injury in the myocardium, in a manner dependent on the presence of circ-0008302 in the ADSC-Exo.

The effects of ADSCs-Exo and circ-0008302 in H2O2-induced cardiomyocytes injury.
A. M6200 cells treated with ADSCs-Exo in the presence of circ-0008302 siRNA or a negative control siRNA for 36 hours, followed by H2O2 for 24 hours. The viability of M6200 cells according to the CCK8 assay. B, C. The levels of apoptosis in the M6200 cells according to the flow cytometry. **P < 0.01, one-way ANOVA.
Effects of ADSC-Exos in Protecting Cardiomyocytes Against Oxidative Damage Through circ-0008302
Because the cardiomyocyte damage caused by H2O2 is due primarily to ROS production, we evaluated whether ADSC-Exos might decrease ROS production through circ-0008302 in H2O2-treated cardiomyocytes. H2O2 treatment resulted in greater generation of intracellular ROS than observed in the control group, whereas this effect was reversed by ADSC-Exo treatment. However, knockdown of circ-0008302 abrogated the effects of ADSC-Exos in decreasing intracellular ROS production (Figure 4A, B). Our results suggested that ADSC-Exos may prevent oxidative damage to cardiac myocytes, in a manner dependent on the presence of circ-0008302.

Detection of ROS production.
A. M6200 cells treated with ADSCs-Exo in the presence of circ-0008302 siRNA or the negative control siRNA for 36 hours, followed by H2O2 treatment for 24 hours. The ROS levels in the M6200 cells according to the flow cytometry analysis. B. Quantitative analysis of the ROS production. **P < 0.01, one-way ANOVA.
Effects of miR-466i-5p and MsrA Expression Levels on the Protective Effects of ADSC-Exos and circ-0008302
Exosomal circRNAs often exert their effects by binding miRNAs in cells. Therefore, we sought to identify molecules downstream of circ-0008302. On the basis of sequencing results, circ-0008302 was predicted to bind miR-466d-5p, miR-669f-3p, miR-466i-5p, miR-466b-3p, and miR-466c-3p, among which miR-466d-5p and miR-466i-5p had multiple binding sites. Previous research has demonstrated that miR-466i-5p plays a crucial role in various pathological processes, including fibrosis [25], inflammation [26, 27], cancer [28], and metabolic disorders [29]. However, its function in cardiovascular diseases has not been investigated, to our knowledge. Therefore, we selected miR-466i-5p for the following experiments. In cardiomyocytes treated with H2O2, the level of miR-466i-5p was greater than that in the control group. Treatment with ADSC-Exos significantly decreased miR-466i-5p levels. However, knockdown of circ-0008302 significantly increased miR-466i-5p levels in cardiomyocytes treated with ADSC-Exos (Figure 5A). The above results indicated that the ADSC-Exos may decrease the expression of miR-466i-5p induced by H2O2 in cardiomyocytes with high expression of circ-0008302.

The role of exosomal circ-0008302 on the MiR-466i-5p targets and MsrA in cardiomyocytes.
A. M6200 cells treated with ADSCs-Exo in the presence of circ-0008302 siRNA or the negative control siRNA for 36 hours, followed by H2O2 treatment for 24 hours. The expression of miR-466i-5p in M6200 cells was evaluated by qRT-PCR. B. The predicted binding site between MsrA 3′-UTR and miR-466i-5p according to the Targetscan database. C. M6200 cells were treated with ADSCs-Exo in the presence of circ-0008302 siRNA or the negative control siRNA for 36 hours, followed by H2O2 treatment for 24 hours. The expression of MsrA in cardiomyocytes was evaluated by western blotting analysis. D. Statistical MsrA expression results, **P < 0.01, one-way ANOVA.
Finally, we explored the effects of the target miR-466i-5p gene. We used data extracted from the Targetscan database to predict the binding of miR-466i-5p to the 3′-UTR of MsrA, an antioxidant factor that inhibits the inflammatory response and negatively regulates the nuclear factor kappa B pathway [30, 31]. Consequently, we validated the effects of miR-466i-5p on MsrA expression, and observed that H2O2 treatment decreased MsrA levels in cardiomyocytes, whereas treatment with ADSC-Exos significantly reversed this effect and improved MsrA expression. Moreover, circ-0008302 knockdown significantly decreased MsrA expression in ADSC-Exo-treated cardiomyocytes (Figure 5C, D). Our results suggested that ADSC-Exos positively regulate MsrA expression in cardiomyocytes, and this effect may depend on the suppression of miR-466i-5p by circ-0008302.
Discussion
Cardiovascular disease is the leading cause of death worldwide; the most severe type is myocardial infarction, which has a poor prognosis [1]. Even after successful percutaneous coronary intervention and surgical treatment, myocardial ischemia-reperfusion can still cause various tissue injuries, such as apoptosis and hypertrophy. ADSCs have been shown to promote the repair and regeneration of damaged tissues [8]. However, the mechanism underlying this process remains unclear. Therefore, in this study, we aimed to investigate the effect of ADSC-Exos on myocardial injury, and to elucidate potential mechanisms, by examining the influence of circRNAs on tissue injury and repair.
Our findings indicated that the delivery of circ-0008302 via ADSC-Exos preserved cardiomyocyte viability, inhibited apoptosis, and decreased ROS production, thus ultimately protecting cardiomyocytes against oxidative injury induced by H2O2. Previous studies have reported the potential efficacy of human umbilical cord mesenchymal stem cells in repairing vascular injury [32, 33]. However, ADSCs are easier to obtain in large quantities from adipose tissue than human umbilical cord mesenchymal stem cells. Moreover, ADSCs can be rapidly expanded in vitro, thus providing a convenient and practical option for cell-based therapy. Exosomes are a crucial component of the paracrine signaling system and serve as important mediators of intercellular signal transduction. They also play critical roles in tissue repair by packaging and delivering various bioactive molecules, including RNAs and proteins. Cell-derived exosomes are safer and more convenient to deliver or store than cells. Consequently, ADSC-Exos are increasingly being used to treat various diseases [34, 35].
In recent years, RNA therapy has emerged as a promising therapeutic option for cardiac regeneration and protection. As endogenous non-coding RNA molecules, circRNAs have been gaining increasing attention because they are resistant to exonuclease-mediated degradation and consequently can confer stable long-term protection. In this study, we analyzed the differential expression of circRNAs between ADSCs and ADSC-Exos, and identified circ-0008302 as a crucial mediator responsible for the protective effect of ADSC-Exos. Furthermore, we observed significant changes in the expression of circ-0007490 and circ-0010012 in the ADSC-Exo group. Unlike circ-0008302, circ-0007490 and circ-0010012 were downregulated in ADSC-Exos. Previous studies have reported that circ0007490 is involved in central nervous system dysfunction [24], whereas circ-0010012 has been identified as a regulatory factor in idiopathic dilated cardiomyopathy [22]. However, the roles of these circRNAs in the pathogenesis of myocardial injury remain unknown and warrant further investigation.
MiRNAs are small (∼22 nt) non-coding RNAs with crucial roles in regulating gene expression by inhibiting mRNA translation [36]. These RNAs are essential in various biological processes, including regulating electrical signals, muscle contraction, heart growth, and morphogenesis [37]. Consequently, numerous studies have demonstrated that changes in miRNA expression profiles are often associated with heart diseases such as myocardial infarction and heart failure [38]. Therefore, miRNAs have emerged as potential therapeutic targets for these conditions [39]. In this study, mechanistic investigations revealed that circ-0008302 targets and inhibits miR-466i-5p expression. Moreover, circ-0008302 has been detected in the forebrain, and its host gene, Ralgps2, directly influences endothelial sprouting during prolonged hypoxic culturing [40, 41]. We further confirmed that ADSC-Exos inhibited the expression of miR-466i-5p by delivering circ-0008302, thus maintaining expression of the miR-466i-5p target gene MsrA in cardiomyocytes subjected to H2O2 damage. Importantly, although miR-466i-5p showed the highest binding score to circ-0008302, other miRNAs, such as miR-466d-5p, miR-669f-3p, miR-466b-3p, and miR-466c-3p, might also potentially bind. Thus, the functions of these other candidates will require careful examination in future studies.
MsrA, a target gene of miR-466i-5p, has been reported to participate in antioxidant defense, thereby protecting the host against tissue damage caused by inflammation or chemicals [30, 31]. Given that an excessive inflammatory response is a common factor driving the pathogenesis of myocardial injury, we speculate that the upregulation of MsrA mediated by circ-0008302 might mitigate myocardial injury by suppressing cardiac inflammation. However, further investigations are required to validate the exact mechanisms of action of MsrA.
Study Limitations
This study has at least two limitations that must be addressed. First, the study involved only in vitro experiments; therefore, the biological characteristics of circ-0008302, miR-466i-5p, and MsrA must be explored in animal models. Second, little research has been conducted on circ-0008302, and further research is necessary to determine which downstream substrates of the MsrA signaling pathway regulate myocardial inflammation and myocardial injury.
Conclusions
ADSC-Exos decrease myocardial injury. Their protective role is mediated by the delivery of circ-0008302 to cardiomyocytes, thereby increasing MsrA expression via the targeting of miR-466i-5p. This study may provide a basis for further exploration of protective effects against myocardial injury and the biological basis for designing potential precision therapy for patients with AMI.