Introduction
Vascular calcification (VC), an abnormal condition affecting the cardiovascular system, is characterized by calcium-phosphate deposition [1]. VC is associated with diminished vascular elasticity, which in turn increases vulnerability to cardiovascular events, diabetes, and chronic kidney disease [2, 3]. Abdominal aortic calcification (AAC), a type of VC, is considered a separate predictor of cardiovascular events and mortality [4]. Previously identified risk factors for AAC include advanced age, smoking, hypertension, and diabetes [5, 6].
Methylmalonic acid (MMA) is commonly used to identify hereditary methylmalonic acidemia and to vitamin B12 sufficiency [7]. MMA is an intermediate metabolite in the catabolism of amino acids and odd-chain fatty acids [8]. MMA accumulation leads to inhibition of several enzymes including methylmalonyl-CoA mutase in the tricarboxylic acid cycle, respiratory chain metabolism, and mitochondrial transport leading to augmented generation of oxidative stress and reactive oxygen species, mitochondrial membrane potential, and abnormal bioenergetics profiling [9]. Diabetes, cardiovascular disease, and aging have all been associated with MMA [10–12].
The advancement of VC is affected by multiple cell communication pathways, such as autophagy, oxidative stress, and mitochondrial dysfunction [13–15]. Recent research has indicated that an abundance of phosphate is associated with impaired mitochondrial function in vascular smooth muscle cells (VSMCs). This impairment manifests as elevated reactive oxygen species, poor ATP generation, and impaired mitochondrial membrane potential [16]. AAC and MMA accumulation are both age-related diseases with similar risk factors. Therefore, elucidating their association is critical to understanding the fundamental underlying mechanisms. However, sufficient research has not been conducted on the link between MMA and AAC. Consequently, this study examined the relationship between serum MMA levels and AAC prevalence among participants ≥40 years of age in the National Health and Nutrition Examination Survey (NHANES).
Materials and Methods
Study Population and Design
NHANES, a cross-sectional sample survey, is performed every 2 years by the National Center for Health Statistics to evaluate the health and nutritional status of Americans of all ages. Data are collected through both interviews and examinations. Trained personnel administer standardized questionnaires to collect data on demographic, socioeconomic, nutritional, and health factors. Qualified medical professionals conduct physical assessments and laboratory tests at mobile examination facilities.
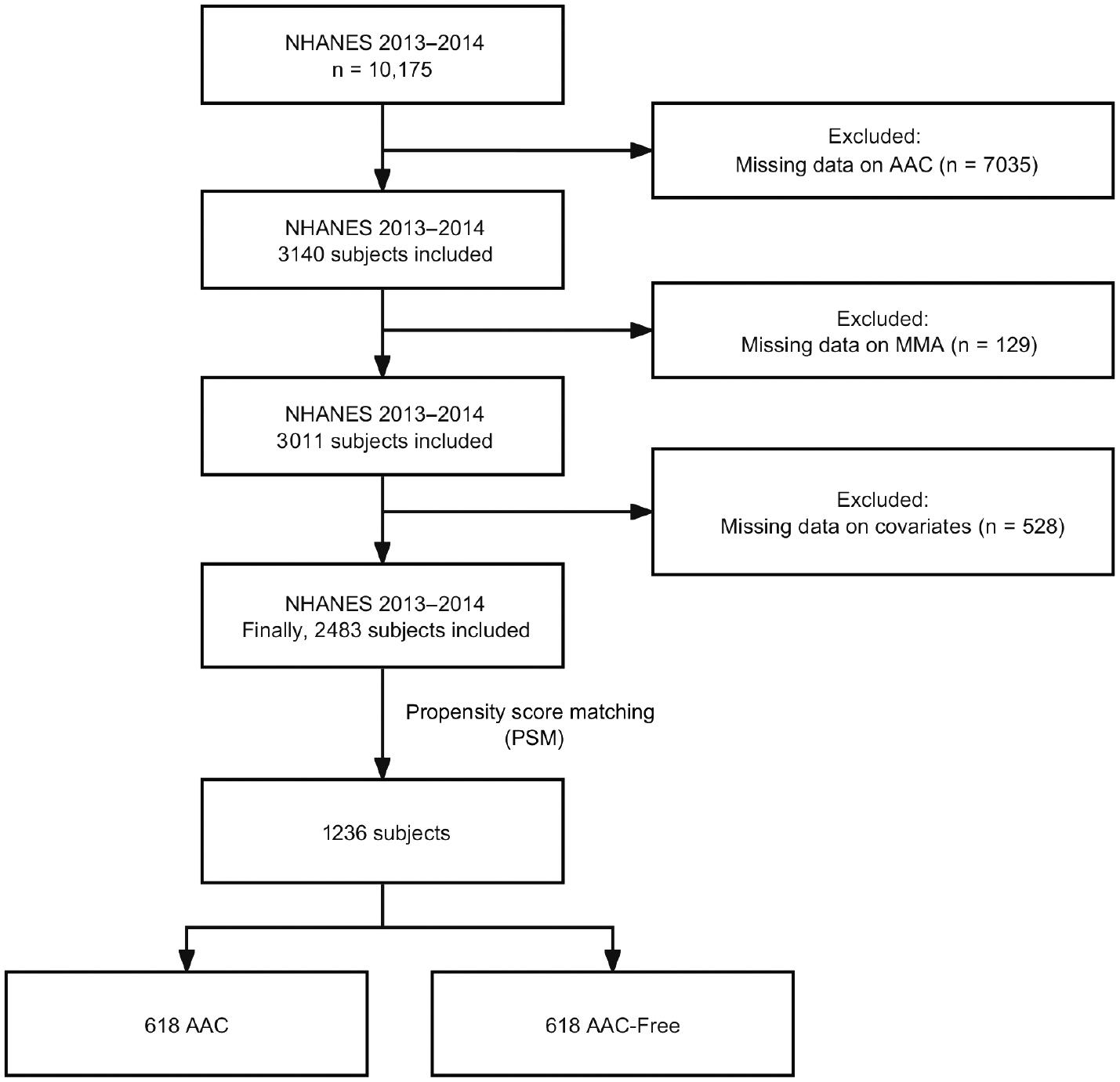
Data on patients with AAC were obtained from the survey conducted from 2013 to 2014. A total of 10,175 participants were included in this study. Any participants with missing data for variables, MMA, or AAC were excluded. The final sample in the cross-sectional analysis comprised 2483 participants. Figure 1 shows the flowchart for the investigation.
Assessment of AAC
The degree of calcification was assessed with the AAC score, which was determined according to lateral spine dual-energy X-ray absorptiometry (DXA) images. NHANES uses DXA because of its low radiation dose, excellent sensitivity, and good accuracy in detecting AAC. The prevalence of AAC was assessed with the established Kauppila score system, which is commonly used for evaluating this measurement [17, 18]. Only individuals 40 years of age or above were eligible to receive DXA scans. The eligibility criteria for the study included not being pregnant, not having used barium for radiographic contrast in the past week, having a weight not exceeding 450 pounds, and not having a Harrington rod in the spine for scoliosis. The AAC score ranged from 0 to 24, and a score of 1 or greater was defined as AAC [6, 19].
Covariates
The basic demographic variables included age, sex, race, and education level. Race was categorized as non-Hispanic White, non-Hispanic Black, or other. Education levels were categorized as less than high school, or high school or above. The formula for calculating body mass index (BMI) was weight in kilograms divided by height in meters squared. A BMI <25 kg/m2 was considered normal weight, whereas a BMI ≥25 kg/m2 was considered overweight. Individuals with a history of smoking at least 100 cigarettes were categorized as smokers. Participants with a medical history of hypertension, diabetes, or coronary artery disease (CAD) were included in our study. Participants who answered affirmatively to the questions “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?” or “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” were classified as having hypertension or diabetes, respectively. Participants who responded affirmatively to the question “Has a doctor or other health professional ever told you that you had coronary heart disease?” were defined as having CAD. In addition, hypertension, diabetes, serum vitamin B12, serum folate, serum total calcium, serum phosphorus, total cholesterol (TC), hemoglobin, high-density lipoprotein cholesterol (HDL-C), serum uric acid, blood urea nitrogen (BUN), urine creatinine, and serum 25(OH)D were included in our study. The Modification of Diet in Renal Disease equation was used for determining the estimated glomerular filtration rate (eGFR) [20]. The NHANES Dietary Data section was used to determine the dietary folic acid and vitamin B12 intake of the participants in the prior 30 days.
Statistical Analysis
Because of the complexity of the sampling strategy in the NHANES investigation, we used sample weights for all statistical analyses in this research. Continuous data and categorical data are presented as medians and percentages, respectively. Previous research has suggested that MMA levels ≥250 nmol/L serve as a diagnostic factor for vitamin B12 deficiency [12]. Therefore, participants were divided into two groups, on the basis of MMA levels ≥250 nmol/L or <250 nmol/L. We adjusted for the confounding effects of age and sex through propensity score matching (PSM) with the R package MatchIt [21]. The Spearman correlation coefficient was used to assess the correlations among all continuous covariates. Univariate and multivariate analyses were performed to investigate the relationship between MMA level and AAC. The multivariate model was controlled for MMA groups, hypertension, smoking status, CAD, HDL-C, serum calcium, and diabetes. The accuracy of the multivariate logistic regression model in assessing AAC predictions was evaluated according to the receiver operating characteristic (ROC) curve. R software (version 4.3.0) was used for all analyses, and a significance threshold of 0.05 was applied for all tests.
Results
Baseline Characteristics
The NHANES cycle from 2013 to 2014 included 10,175 participants. After exclusion of individuals with incomplete AAC information (n = 7035), our analysis further excluded those with missing MMA information (n = 129). In addition, we excluded 528 participants with missing covariate data. Finally, a total of 2483 individuals were registered for participation in this study. A summary of the attributes of the excluded individuals is provided in Supplementary Table 1, including an overview of their baseline information. A summary of the baseline characteristics of the included participants is provided in Table 1. Compared with individuals with low MMA levels, those with high MMA levels were more likely to be older; to be non-Hispanic White; and to have hypertension, diabetes, low serum B12, high uric acid, high BUN, high serum creatinine, low eGFR, or high prevalence of AAC. A total of 2483 participants were registered in this research, comprising 732 with AAC and 1751 without AAC. In comparison to individuals without AAC, those with AAC were more likely to be older; to be non-Hispanic White; to have hypertension or diabetes; to be smokers; and to have CAD, high serum folate, high uric acid, high BUN, high serum creatinine, low eGFR, low HDL-C, low vitamin B12 intake, or elevated MMA levels (Supplementary Table 2).
Baseline Characteristics of Participants from NHANES 2013–2014 by Serum MMA Level.*
| Characteristic | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall, n = 2483 † | Low MMA, n = 2120 † | High MMA, n = 363 † | P value ‡ | Overall, n = 1236 † | Low MMA, n = 1020 † | High MMA, n = 216 † | P value ‡ | |
| Methylmalonic acid (nmol/L) | 157 (122, 204) | 148 (117, 180) | 319 (274, 405) | <0.001 | 160 (125, 210) | 149 (121, 180) | 318 (274, 407) | <0.001 |
| Age (years) | 56 (48, 66) | 56 (47, 65) | 63 (53, 74) | <0.001 | 62 (53, 70) | 60 (52, 70) | 68 (58, 75) | 0.001 |
| Female (%) | 1316 (53%) | 1135 (53%) | 181 (53%) | 0.9 | 630 (51%) | 529 (51%) | 101 (51%) | 0.9 |
| Race (%) | 0.005 | 0.012 | ||||||
| Non-Hispanic White | 1162 (73%) | 951 (72%) | 211 (80%) | 628 (77%) | 497 (76%) | 131 (82%) | ||
| Non-Hispanic Black | 469 (9.4%) | 429 (10%) | 40 (5.3%) | 217 (7.9%) | 196 (8.6%) | 21 (4.3%) | ||
| Other | 852 (17%) | 740 (18%) | 112 (14%) | 391 (15%) | 327 (16%) | 64 (14%) | ||
| Education (%) | 0.5 | 0.6 | ||||||
| Less than high school | 502 (14%) | 418 (13%) | 84 (15%) | 259 (14%) | 208 (14%) | 51 (15%) | ||
| High school or above | 1981 (86%) | 1702 (87%) | 279 (85%) | 977 (86%) | 812 (86%) | 165 (85%) | ||
| Body mass index (kg/m2) | 0.15 | 0.008 | ||||||
| Normal weight | 675 (27%) | 565 (26%) | 110 (31%) | 321 (24%) | 253 (22%) | 68 (33%) | ||
| Overweight | 1808 (73%) | 1555 (74%) | 253 (69%) | 915 (76%) | 767 (78%) | 148 (67%) | ||
| Hypertension (%) | 1174 (44%) | 975 (42%) | 199 (54%) | 0.001 | 652 (49%) | 527 (48%) | 125 (58%) | 0.018 |
| Diabetes (%) | 397 (12%) | 319 (11%) | 78 (19%) | <0.001 | 225 (15%) | 172 (13%) | 53 (24%) | 0.003 |
| Smoker (%) | 1126 (45%) | 947 (44%) | 179 (49%) | 0.2 | 618 (50%) | 496 (48%) | 122 (58%) | 0.071 |
| CAD (%) | 135 (5.0%) | 102 (4.8%) | 33 (6.6%) | 0.10 | 84 (6.4%) | 59 (6.1%) | 25 (8.1%) | 0.14 |
| Serum B12 (pg/mL) | 518 (385, 720) | 538 (407, 742) | 373 (281, 546) | <0.001 | 515 (384, 736) | 537 (409, 755) | 385 (276, 568) | <0.001 |
| Serum folate (ng/mL) | 20 (13, 29) | 20 (13, 29) | 18 (11, 29) | 0.3 | 20 (13, 29) | 20 (13, 29) | 20 (12, 31) | 0.8 |
| Serum calcium (mg/dL) | 9.40 (9.20, 9.70) | 9.40 (9.20, 9.70) | 9.50 (9.20, 9.70) | 0.12 | 9.40 (9.20, 9.70) | 9.40 (9.20, 9.70) | 9.60 (9.30, 9.80) | 0.033 |
| Serum phosphorus (mg/dL) | 3.80 (3.40, 4.20) | 3.80 (3.40, 4.20) | 3.80 (3.40, 4.26) | 0.4 | 3.80 (3.40, 4.20) | 3.80 (3.40, 4.20) | 3.80 (3.30, 4.10) | 0.9 |
| Uric acid (mg/dL) | 5.30 (4.40, 6.20) | 5.30 (4.40, 6.20) | 5.60 (4.60, 6.60) | 0.006 | 5.40 (4.50, 6.30) | 5.30 (4.50, 6.20) | 5.60 (4.70, 6.40) | 0.050 |
| Total cholesterol (mg/dL) | 193 (166, 220) | 193 (167, 220) | 192 (162, 219) | 0.4 | 191 (164, 220) | 192 (166, 221) | 186 (154, 219) | 0.088 |
| BUN (mg/dL) | 13 (11, 17) | 13 (11, 16) | 15 (12, 20) | <0.001 | 14 (11, 18) | 14 (11, 17) | 16 (12, 21) | 0.004 |
| Serum creatinine (mg/dL) | 0.88 (0.75, 1.02) | 0.87 (0.74, 1.01) | 0.96 (0.77, 1.18) | 0.002 | 0.89 (0.76, 1.05) | 0.87 (0.76, 1.02) | 0.97 (0.77, 1.22) | <0.001 |
| Urine creatinine (mg/dL) | 87 (48, 141) | 88 (49, 142) | 83 (45, 134) | 0.7 | 87 (51, 136) | 88 (52, 136) | 82 (45, 133) | 0.3 |
| eGFR (mL/min/1.73 m3) | 79 (67, 90) | 80 (68, 90) | 70 (54, 83) | <0.001 | 77 (65, 88) | 78 (67, 89) | 68 (52, 81) | <0.001 |
| HDL-C (mg/dL) | 52 (43, 65) | 52 (43, 65) | 51 (41, 64) | 0.5 | 52 (43, 63) | 52 (43, 63) | 50 (42, 61) | 0.6 |
| Hemoglobin (g/dL) | 14.10 (13.30, 15.00) | 14.10 (13.30, 15.10) | 14.00 (13.00, 14.80) | 0.10 | 14.30 (13.30, 15.00) | 14.30 (13.30, 15.10) | 14.25 (13.10, 14.70) | 0.2 |
| Serum 25(OH)D (nmol/L) | 73 (57, 91) | 74 (58, 91) | 71 (51, 92) | 0.5 | 75 (58, 92) | 75 (59, 92) | 71 (49, 93) | 0.6 |
| Folic acid intake (mcg) | 134 (77, 220) | 132 (76, 220) | 138 (83, 223) | 0.3 | 133 (74, 219) | 133 (74, 215) | 132 (74, 244) | 0.5 |
| Vitamin B12 intake (mcg) | 3.9 (2.5, 5.9) | 3.9 (2.5, 6.1) | 3.6 (2.3, 5.5) | 0.093 | 3.8 (2.5, 5.9) | 3.8 (2.5, 6.0) | 3.5 (2.3, 5.8) | 0.5 |
| AAC (%) | 732 (28%) | 575 (26%) | 157 (43%) | <0.001 | 618 (50%) | 497 (48%) | 121 (59%) | 0.002 |
*Data are shown as median ± interquartile range. PSM: propensity score matching; MMA, methylmalonic acid; CAD, coronary artery disease; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; AAC, abdominal aortic calcification.
†Median (IQR) for continuous; n (%) for categorical variables.
‡Wilcoxon rank-sum test for complex survey samples; chi-squared test with Rao and Scott’s second-order correction.
PSM minimized the influence of potential confounding bias between groups. Using age and sex as variables, we applied a logistic regression model. The two sets were paired with a nearest neighbor matching algorithm with a caliper of 0.05 in a one-to-one ratio. After PSM, the age difference between individuals with and without AAC significantly decreased, and the age became comparable between groups. No substantial variations in sex, education, smoking status, and CAD were observed between the high and low MMA groups after PSM. Moreover, no differences in the levels of serum folate, serum phosphorus, TC, urine creatinine, HDL-C, hemoglobin, 25-hydroxyvitamin D (25(OH)D), folic acid intake, and vitamin B12 intake were observed between groups. Notable differences in BMI and serum calcium were observed between cohorts after PSM. Participants diagnosed with AAC, compared with those without AAC, had a higher likelihood of having hypertension, being smokers, and having CAD or elevated MMA levels after PSM. The statistical results indicated a strong relationship between MMA level and AAC incidence.
Serum MMA and AAC
To investigate the correlation between the covariates and serum MMA levels, we conducted Spearman correlation analysis (Figure 2). Significant associations of MMA with uric acid, TC, BUN, serum creatinine, and eGFR were observed, whereas no associations were found between MMA and the dietary intake of vitamin B12 or folic acid. Notably, although a negative correlation was observed between MMA and vitamin B12, it was not statistically significant. Table 2 displays the findings of both univariate and multivariate logistic regression analyses investigating the relationship between MMA and AAC. The univariate analysis indicated that hypertension, smoking, HDL-C, CAD, and MMA were significantly associated with AAC. The multivariate logistic regression analysis results are shown in Table 2. Although serum calcium and diabetes did not show statistical significance in the univariate regression, these variables were included in the multifactorial regression, because they have been linked to AAC incidence in prior research [22, 23]. After correction for diabetes, HDL-C, CAD, hypertension, smoking, and serum calcium, elevated MMA was substantially associated with the incidence of AAC (OR: 1.38, 95% CI: 1.01–1.90, P = 0.046). In addition, smoking remained a risk factor for AAC even after adjustment for the effects of confounding variables (OR: 1.84, 95% CI: 1.04–3.27, P = 0.039). On the basis of an area under the ROC curve of 0.612 (95% CI: 0.580–0.643), the logistic regression model used in this study demonstrated an ability to diagnose AAC (Figure 3).
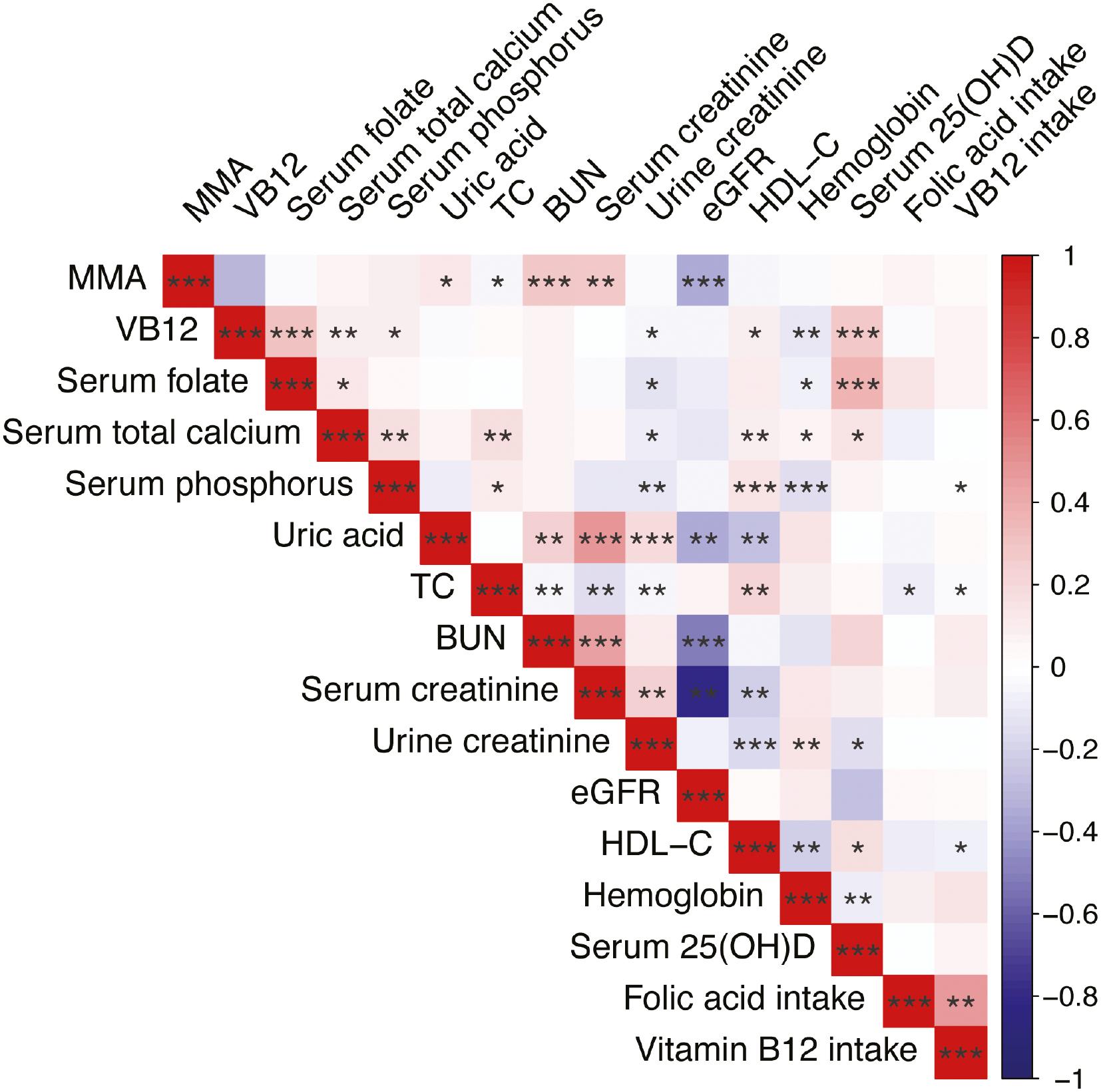
Spearman Correlation of Continuous Covariates.
MMA, continuous methylmalonic acid; VB12, vitamin B12; TC, total cholesterol; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; VB12 intake, vitamin B12 intake. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001).
Univariate Logistic Regression and Multivariate Logistic Regression Analyses of AAC Prevalence after PSM.
| Characteristic | Univariate regression | Multivariate regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Race | ||||||
| Non-Hispanic White | — | — | ||||
| Non-Hispanic Black | 0.92 | 0.52, 1.62 | 0.764 | |||
| Other | 0.95 | 0.61, 1.48 | 0.820 | |||
| High school or above | 0.79 | 0.51, 1.22 | 0.265 | |||
| Body mass index | 0.75 | 0.52, 1.09 | 0.126 | |||
| Hypertension | 1.66 | 1.11, 2.49 | 0.018 | 1.53 | 1.00, 2.34 | 0.051 |
| Diabetes | 1.36 | 0.85, 2.18 | 0.182 | 1.04 | 0.67, 1.62 | 0.8 |
| Smoker | 1.94 | 1.16, 3.26 | 0.015 | 1.84 | 1.04, 3.27 | 0.039 |
| Serum B12 | 1.00 | 1.00, 1.00 | 0.961 | |||
| Serum folate | 1.00 | 0.99, 1.01 | 0.510 | |||
| Serum calcium | 1.28 | 0.73, 2.25 | 0.362 | 1.17 | 0.62, 2.22 | 0.6 |
| Serum phosphorus | 0.90 | 0.73, 1.13 | 0.342 | |||
| Uric acid | 1.05 | 0.95, 1.17 | 0.285 | |||
| Total cholesterol | 1.00 | 1.00, 1.00 | 0.827 | |||
| HDL-C | 0.99 | 0.98, 1.00 | 0.034 | 0.99 | 0.98, 1.00 | 0.074 |
| Hemoglobin | 1.02 | 0.93, 1.12 | 0.623 | |||
| Serum 25(OH)D | 1.00 | 0.99, 1.00 | 0.544 | |||
| Urine creatinine | 1.00 | 1.00, 1.00 | 0.242 | |||
| BUN | 1.01 | 0.99, 1.03 | 0.361 | |||
| Serum creatinine | 1.13 | 0.70, 1.82 | 0.597 | |||
| Coronary heart disease | 2.02 | 1.24, 3.29 | 0.008 | 1.59 | 0.90, 2.81 | 0.10 |
| Folic acid intake | 1.00 | 1.00, 1.00 | 0.186 | |||
| Vitamin B12 intake | 0.97 | 0.93, 1.00 | 0.061 | |||
| eGFR | 1.00 | 0.99, 1.01 | 0.542 | |||
| High MMA | 1.56 | 1.20, 2.03 | 0.003 | 1.38 | 1.01, 1.90 | 0.046 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; PSM: propensity score matching; MMA, methylmalonic acid; CAD, coronary artery disease; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; AAC, abdominal aortic calcification.
Bold values indicate variables that were significant in the regression model (P value < 0.05).
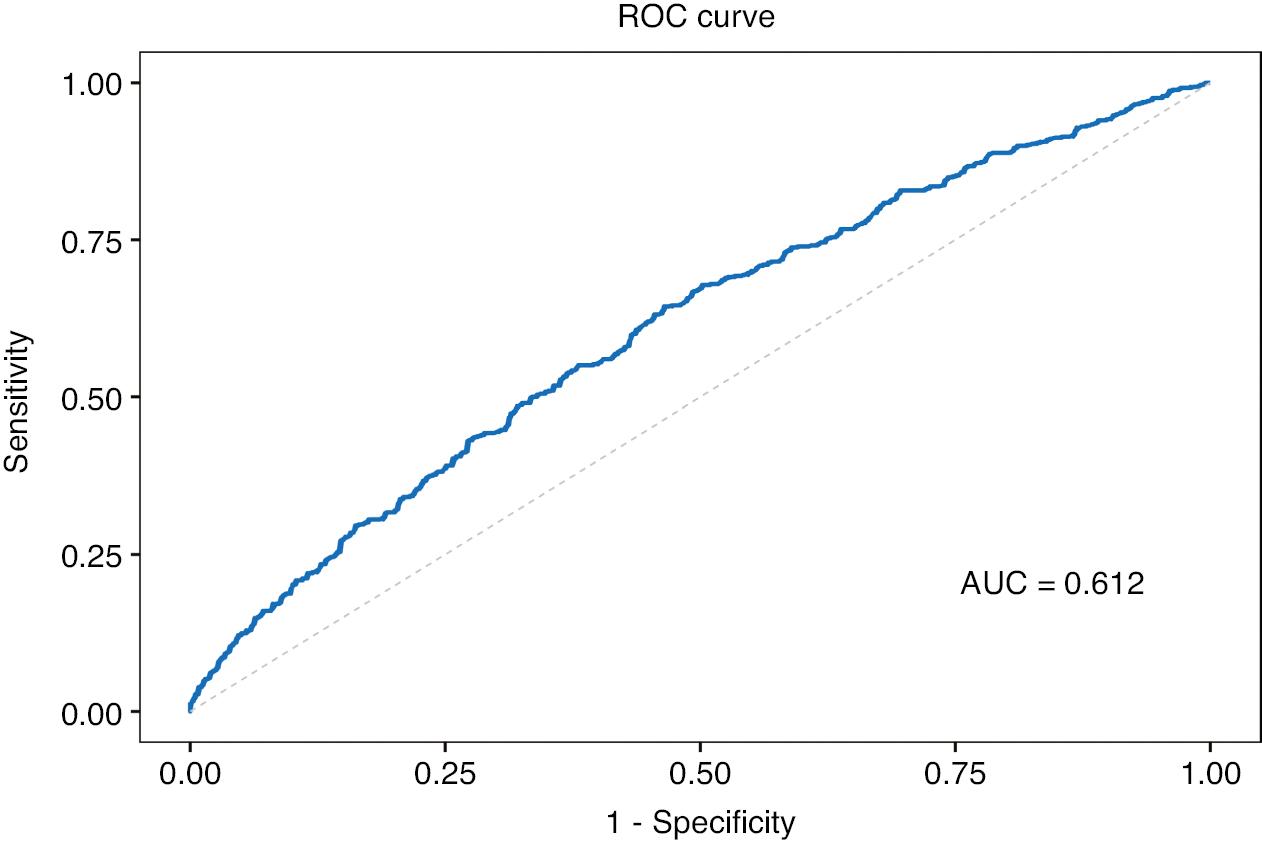
Analysis of the Receiver Operating Characteristic (ROC) Curve to Forecast AAC and its Area under the Curve (ACU) Value.
Furthermore, 732 participants with a Kauppila score ≥1 were included in this research to examine the relationship between MMA level and AAC severity. Severe abdominal aortic calcification was defined by a Kauppila score >6 [24]. Supplementary Table 3 provides a summary of the individuals’ baseline characteristics. A correlation was observed between higher MMA levels and greater probability of having severe abdominal aortic calcification. Only significant covariates from the univariate regressions were included, because of sample size limitations. Interestingly, multivariate logistic regression analysis revealed no significant correlation between MMA and severe abdominal aortic calcification, as shown in Supplementary Table 4.
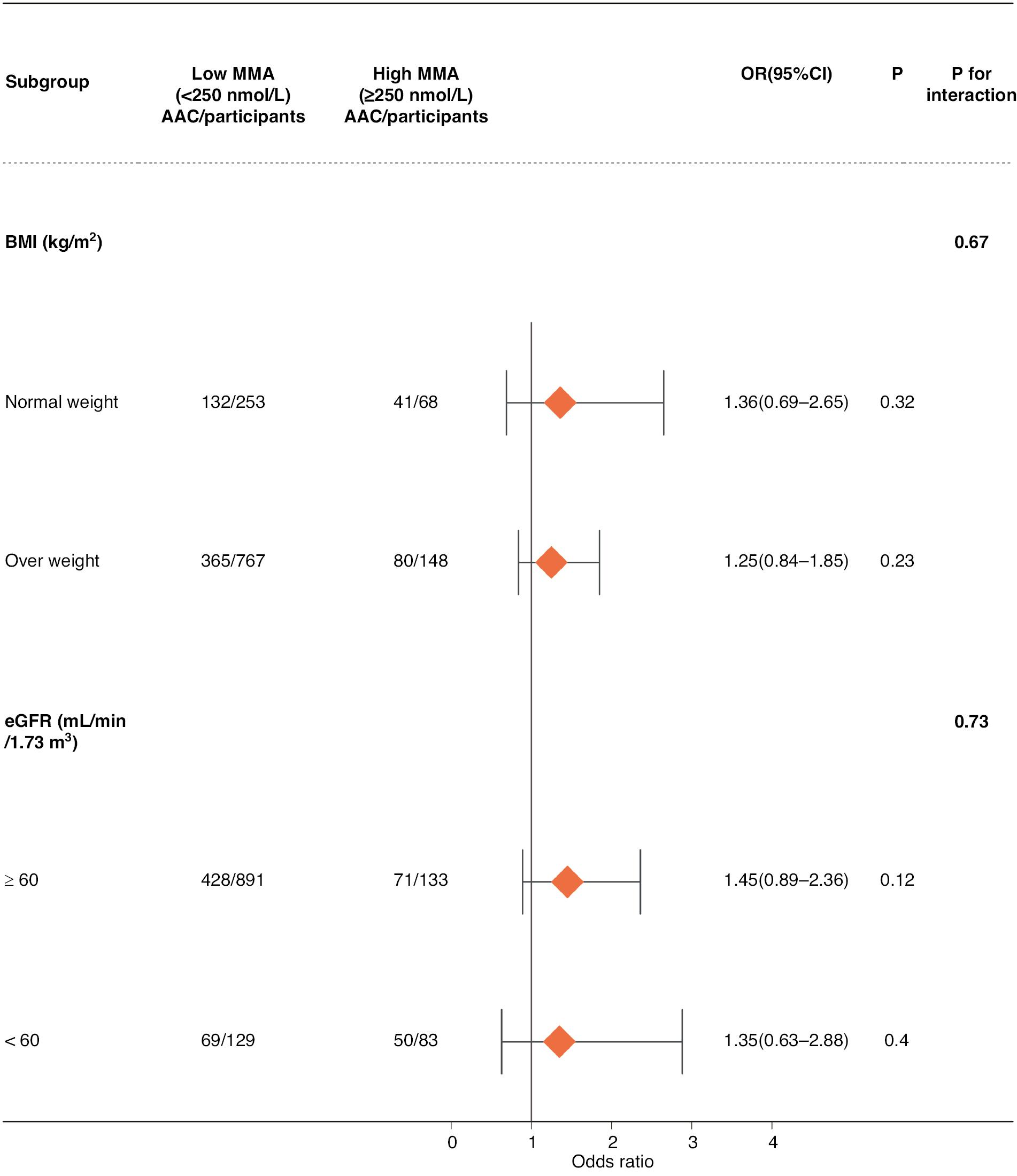
Subgroup Analysis
To investigate the link between MMA and AAC in greater detail, we performed analysis according to BMI and eGFR subgroups (Figure 4). Participants were divided into two categories according to a threshold value of 60 mL/min/1.73 m2 for eGFR. The subgroup analysis revealed no remarkable association between MMA and the occurrence of AAC across subgroups defined by BMI and eGFR. BMI and eGFR did not significantly affect the association between MMA and AAC.
Discussion
Serum MMA levels ≥250 nmol/L were found to substantially correlate with AAC, a severe vascular illness that frequently occurs with insulin resistance, diminished osteocalcin, diminished bone mineral density, and cardiovascular disease [25–27]. MMA is a biomarker of metabolic product accumulation associated with aging, and may trigger cancer progression and infiltration via metabolic reprograming [28]. In addition, a cross-sectional study has indicated that elevated levels of MMA derived from mitochondria significantly correlate with both overall mortality and cardiovascular-related deaths [12]. Furthermore, a notable interaction has been reported between MMA and impaired renal function [29]. However, to our knowledge, no previous evidence has indicated an association between MMA and the prevalence of AAC. Therefore, our study provides novel evidence that MMA is associated with AAC.
The univariate analysis indicated that hypertension was positively associated with AAC occurrence. Calcium accumulation has been reported to contribute to the development of arterial rigidity, which in turn adversely affects blood pressure because of decreased potential energy in elastic arteries [30]. Furthermore, vascular tissue remodeling caused by hypertension has been suggested to provide ideal circumstances for calcium deposition [31]. VSMC migration, apoptosis, fibrosis and inflammation production due to activation of the renin–angiotensin system in hypertensive patients leads to vascular remodeling [32]. CAD and HDL-C are both independent predictors of AAC [33, 34]. Both CAD and AAC tend to occur more frequently with advancing age, and calcium is often present in atherosclerotic plaques [35]. These findings have implied a potential correlation in a shared calcification process between AAC and CAD. In addition, HDL-C is a risk factor common to CAD and AAC, and has been reported to be negatively associated with vascular disease occurrence [36, 37]. Furthermore, smoking, a common risk factor for cardiovascular disease, is also strongly associated with AAC, in agreement with our findings [38]. Cigarette smoking negatively affects VSMCs through the activation of autophagy and ferroptosis pathways mediated by diverse signals [39, 40]. Smoking might alter the vascular tissue and lead to calcification. Therefore, further investigation of the mechanisms linking hypertension, CAD, HDL-C, tobacco use, and AAC is warranted.
Our study found a significant interaction between MMA and AAC, thus suggesting a potential link between them. However, the precise cause of this link remains unclear. Studies have suggested that increased MMA levels impede the energy production process in mitochondria, and elevated formation of intracellular free radicals further contributes to mitochondrial harm [8]. Moreover, oxidative stress and mitochondrial dysfunction are known to play important roles in the development of AAC. In addition, calcification is significantly influenced by inflammation [16]. Previous studies have revealed increases in the levels of tumor necrosis factor (TNF), interleukin-1β (IL-β), and cyclooxygenase 2 (COX-2) after MMA injection [41, 42]. These findings suggest a clear association of MMA accumulation with neuroinflammation and renal inflammation. Therefore, examining the correlation between MMA buildup and the occurrence of inflammation is crucial.
Diabetes increases the risk of AAC and the likelihood of VC development [16, 43]. This increased risk is mediated through inflammatory, angiogenesis, and endothelial dysfunction pathways [44]. Interventions aimed at improving these pathways have the potential to enhance vascular health in people with diabetes. Interestingly, however, our univariate and multivariate logistic regressions did not find a significant difference between diabetes and AAC. According to a prior study, pregnant women with rather than without vitamin B12 deficiency are more likely to have diabetes [45], thus suggesting a potential relationship between MMA accumulation and diabetes. However, additional investigations are required to examine this correlation among diverse populations.
Multiple factors must be considered in the interpretation of our research findings. First, the cross-sectional study design prevents a definitive cause-effect relationship between MMA and AAC from being deduced. Prospective cohort studies are required to elucidate causality and to better observe the long-term effects of exposure factors on outcomes. Furthermore, fundamental scientific research is needed to examine the biological correlation between MMA and AAC. Second, a larger sample size is required, because the use of PSM decreased the sample size.