Introduction
Tricuspid regurgitation (TR), the most prevalent right heart valvular lesion, affects 65–85% of the population worldwide [1, 2]. Mild TR is usually benign and is considered a normal variant. However, moderate or severe TR is typically pathologic, and is often associated with leaflet abnormalities and/or annular dilation, thus leading to irreversible myocardial damage, and substantial morbidity and mortality [3]. Moderate or severe TR has been estimated to affect approximately 0.55% of the general population and to have an incidence as high as 4% in people 75 years of age or older [4]. Managing these patients is challenging, because they often do not respond well to guideline-directed optimal medical therapy. Moreover, surgery is associated with a high perioperative mortality rate in patients with moderate or severe TR, particularly those with right ventricular dysfunction and/or hepatorenal dysfunction due to chronic venous hypertension. According to American guidelines, tricuspid valve (TV) surgery is typically limited to patients with severe TR at the time of left heart cardiac surgery (class I, level C) [5]. On the basis of European guidelines, TV surgery is recommended for patients with severe TR undergoing left-sided valve surgery (class I, level C for primary TR; class I, level B for secondary TR), and for symptomatic patients with isolated severe primary TR and without severe right ventricular dysfunction (class I, level C) [6]. Transcatheter TV edge-to-edge repair (T-TEER) has emerged as a safe and potentially effective technique for decreasing TR, thereby expanding treatment options for patients with severe TR. European guidelines suggest that transcatheter TV interventions may be a possible treatment for patients with secondary severe TR when cardiac surgery poses a prohibitive risk, particularly at experienced heart valve centers (class IIb, level C) [6]. This article provides a comprehensive review of the current state of T-TEER for severe TR.
Anatomy of the TV Complex
The TV is situated between the right atrium (RA) and the right ventricle (RV), and typically has a normal orifice area of 7–9 cm2 [7, 8]. The TV apparatus comprises the annulus, leaflets, chordae tendinae, and papillary muscles [8]. The TV annulus is a highly dynamic, three-dimensional (3D) structure that often has an asymmetric oval- or D-shaped appearance [9–11]. The TV annulus tends to become more planar in patients with severe TR than normal individuals. The anterior papillary muscle provides chordal support for both the anterior and posterior leaflets. The posterior papillary muscle provides chordal support to the posterior and septal leaflets. The septal papillary muscle exhibits variability; it may be small, may exist in multiple forms, or may even be absent [8, 12]. The TV typically consists of three leaflets: the anterior leaflet, posterior leaflet, and septal leaflet. The anterior leaflet is generally the largest of the three leaflets and extends the farthest radially, thus providing a large surface area and the greatest range of motion [13]. The posterior leaflet is characterized by multiple scallops and is the shortest circumferentially. The septal leaflet is the shortest in the radial direction and has the least mobility; it attaches directly to the TV annulus above the interventricular septum and connects to the septal wall through short chordae [14].
With deeper understanding of the variability in TV leaflet morphology, a novel classification scheme for TV leaflets has recently been proposed [15]. This classification is based on observations from the transgastric short-axis view of transesophageal echocardiography (TEE). This classification identifies four TV morphology types: 1) type I, consisting of the typical configuration with three leaflets (found in 54% of patients); 2) type II, consisting of two leaflets, with the anterior and posterior leaflets fused (5% of patients); 3) type IIIA, characterized by the presence of four leaflets, two of which are anterior (found in 3% of patients); 4) type IIIB, which has four leaflets, similarly to type IIIA, but includes two posterior leaflets (32% of patients); 5) type IIIC, which also features four leaflets, two of which are septal (4% of patients); and 6) type IV, which includes TV configurations with more than four leaflets (found in 2% of patients).
Classification of TR
The classification of TR is typically divided into primary (leaflet pathology), secondary (non-leaflet pathology), and cardiac implantable electronic device (CIED)-induced TR (Table 1) [16, 17].
Classification of Tricuspid Regurgitation.
| Classification | Subgroup | Etiology |
|---|---|---|
| Primary TR (5–10%) | Congenital | Ebstein’s anomaly |
| Leaflet clefts | ||
| Acquired | Infective endocarditis | |
| Leaflet infiltration (rheumatic, carcinoid) | ||
| Trauma | ||
| Degenerative | Prolapse | |
| Flail | ||
| Secondary TR (80%) | Atrial | Atrial fibrillation |
| Heart failure with preserved ejection fraction | ||
| Ventricular | Pulmonary hypertension | |
| Left heart disease | ||
| Right ventricular dysfunction | ||
| CIED-induced TR (10–15%) | Primary | Direct interaction of the lead on the leaflets |
| Secondary | Functional etiology related remodeling |
TR, tricuspid regurgitation; CIED, cardiac implantable electronic device.
The primary causes of TR are responsible for a relatively small percentage of cases, typically approximately 5–10%. These primary causes of TR can be categorized into congenital, acquired, and degenerative etiologies. Congenital TR includes conditions such as Ebstein’s anomaly and tricuspid atresia [18, 19]. Acquired TR can result from TV endocarditis, rheumatic or carcinoid TV disease, and traumatic rupture of the TV apparatus [20, 21]. Degenerative diseases include conditions caused by TV prolapse or flail of the TV leaflets.
Secondary TR accounts for approximately 80% of TR cases [22]. Leaflet malcoaptation occurs because of abnormalities in the RA, tricuspid annulus, or RV. Secondary TR can be further categorized into atrial TR and ventricular TR [17, 23, 24]. Atrial secondary TR is an exclusive diagnosis, typically defined by the presence of longstanding or permanent atrial fibrillation with the absence of leaflet abnormality, left ventricular ejection fraction dysfunction, pulmonary hypertension, left-sided valve disease, and CIED [25]. In atrial secondary TR, dilation of the tricuspid annulus and the RA prevents proper coaptation of the TV leaflets, thereby leading to regurgitation [26, 27]. Ventricular secondary TR is often seen in patients with pulmonary hypertension, either pre-capillary or post-capillary [28], and can also occur in patients with primary right ventricular cardiomyopathies, right ventricular myocardial infarction, and certain arrhythmias. Other contributing factors include left ventricular dilatation, left ventricular dysfunction, or concomitant left-sided valvular disease. This form of TR is characterized by septal leaflet tethering and improper coaptation.
CIED-induced TR was initially classified as a subgroup of primary TR. However, as research has progressed, the distinct features of CIED-induced TR have become more apparent. This type of TR exhibits characteristics of both primary and secondary TR, and can be classified as lead-related mechanical interference on TV coaptation, pacing induced TR, or TV dysfunction following lead extraction [29, 30]. Moreover, the epidemiological features and management of CIED-induced TR differ from those in other classifications [31]. TR occurs in approximately 25–29% of patients with permanent pacemakers [32]. Given the current population aging, increased demand for device implantation, and device-related complications associated with lead extraction, the number of patients with CIED-induced TR is expected to continue to rise [33, 34].
Severity of TR
Transthoracic echocardiography is the most commonly used diagnostic imaging method for assessing TR severity. This assessment involves qualitative, semiquantitative, and quantitative evaluations, and should ideally be performed under optimal conditions, including normal blood volume and systemic blood pressure, stable diuretic therapy, and suitable pulmonary pressure (end-expiration) [35]. The American guidelines recommend a multiparametric and hierarchical approach for assessing TR severity, according to a three-grade scheme (mild [1+], moderate [2+], and severe [3+]) [36].
In general, a vena contracta width ≥7 mm, effective regurgitant orifice area (EROA) ≥40 mm2, and regurgitant volume ≥45 mL, qualify as “severe” TR [36, 37]. However, the term “severe” TR does not capture the severity of TR and the therapeutic effects in patients enrolled in early feasibility trials of transcatheter devices. The SCOUT trial, for instance, has found a mean decrease in quantitative EROA of −0.22 ± 0.29 mm2, shifting from “severe TR” to “severe TR” (0.85 ± 0.22 mm2 vs. 0.63 ± 0.29 mm2). This decrease was associated with an increase in forward stroke volume, and resulted in significant improvements in quality of life [38]. The results of the SCOUT trial have indicated differing prognosis among “severe” grades of TR, thus emphasizing the importance of defining these grades in determining prognosis. In 2017, Hahn et al. proposed a new grading scheme including two additional grades (“massive” and “torrential”) [39]. Recent evidence-based European guidelines suggest further subclassifying “severe [3+]” into “severe [3+], massive [4+], and torrential [5+]” in patients in whom transcatheter interventions are being considered, because this extended grading scheme may have prognostic value [6, 40, 41]. The extended grading scheme is detailed in Table 2.
Echocardiographic Criteria for Grading Tricuspid Regurgitation.
| Mild [1+] | Moderate [2+] | Severe [3+] | Massive [4+] | Torrential [5+] | |
|---|---|---|---|---|---|
| Qualitative parameters | |||||
| Tricuspid morphology | Normal or mildly abnormal | Moderately abnormal | Severely abnormal (flail leaflet, large coaptation gap, marked tethering) | ||
| Color-flow jet area | Small, narrow, central | Moderate central | Large central, or eccentric, wall impinging | ||
| Semiquantitative parameters | |||||
| VC width, mm | <3 | 3–6.9 | 7–13.9 | 14–20.9 | ≥21 |
| PISA radius, mm | ≤5.4 | 5.5–8.9 | ≥9 | ||
| Quantitative parameters | |||||
| EROA, cm2 | <20 | 20–39 | 40–59 | 60–79 | ≥80 |
| Regurgitant volume, mL | <30 | 30–44 | 45–59 | 60–74 | ≥75 |
VC, vena contracta; PISA, proximal isovelocity surface area; EROA, effective regurgitant orifice area.
T-TEER in TR
A substantial number of patients currently have inoperable severe TR. For these patients, transcatheter TV interventions have emerged as alternative treatments. These treatments encompass various approaches, including annuloplasty and coaptation devices, and transcatheter TV replacement [42]. Coaptation devices are specifically designed to decrease TR by using clips on the leaflets or by occupying the regurgitant orifice [43, 44]. In recent years, a range of coaptation devices have been developed and have shown promising early results. Among these devices, T-TEER is currently the most widely used for interventional TR treatment in high-risk surgical patients with symptomatic severe TR. For patients with severe TR and heart failure with preserved ejection fraction, decreased RV volume overload and improved biventricular interaction and physiology have been observed after T-TEER [45].
Patient Selection for T-TEER
The T-TEER approach is suitable for most patients with severe TR. However, in some cases, optimal TR decreases to the level of moderate or improved outcomes might not be achieved. Several risk factors, in addition to anatomical considerations, have been associated with less favorable prognosis after T-TEER. These risk factors include RV dysfunction, pulmonary hypertension, atrial fibrillation, cardiohepatic syndrome, chronic kidney disease, and the presence of pacemaker/defibrillator leads with leaflet impingement [46–48]. The ideal candidates for T-TEER are patients with primary TR characterized by prolapse only (no flail) or secondary TR with normal leaflet mobility. A post hoc analysis of a prospective cohort has found that T-TEER is effective and safe in decreasing TR in patients with primary and secondary TR [49]. The results from the TriValve registry have indicated effective decreases in TR in both atrial and ventricular secondary TR after T-TEER. Furthermore, patients with atrial secondary TR showed better survival than those with ventricular secondary TR at the 12-month follow-up [50].
The suitable anatomical structure of TV includes leaflet lengths exceeding 7 mm, a coaptation gap between 3 and 7 mm, and a central TR jet within the anteroseptal commissure [22, 51]. Notably, 30-day follow-up data from the bRIGHT study have suggested that T-TEER achieves similar TR decreases among patients with coaptation gaps of <7 mm, 7–10 mm, and >10 mm [52]. Moreover, patients without cardiac implantable electronic devices are preferred for this procedure. Adequate TEE windows are necessary for visualizing the leaflets. Favorable indications also include normal to mildly diminished RV function, normal to mildly dilated RV, normal peak and mean pulmonary arterial pressure, normal transpulmonary gradient, tricuspid annular plane systolic excursion/tricuspid annular plane systolic excursion ratio exceeding 0.41, and normal renal and liver function [22, 41, 42, 51, 53]. Moreover, a sub-analysis of a monocentric, prospective observational study has indicated that T-TEER may be a feasible and effective approach for decreasing TR in patients after heart transplantation [54].
CIED-induced TR is often considered a relative contraindication for T-TEER. Some cases of CIED-induced TR are attributed to pacemaker leads. Lead-related TR can be caused by damage to the valvular apparatus, lead-related mechanical tethering, lead entanglement in the sub-valvular apparatus, and pacing-induced cardiomyopathy [55, 56]. A substantial limitation of T-TEER in patients with CIEDs is that implantation may result in lead entrapment or “jailing.” Assessing lead location and its effects on TR etiology in this patient population is crucial. Management decisions should be made on the basis of the risk assessment and therapeutic benefit in patients with existing CIED leads and TR. Data regarding the use of T-TEER in patients with CIEDs are limited. Recently, a retrospective study has indicated procedural success, and comparable intrahospital outcomes of T-TEER between patients with or without CIEDs [57]. Prospective clinical studies are highly warranted to demonstrate the feasibility and efficacy of T-TEER in patients with CIEDs.
T-TEER Devices
Currently, two types of devices are available for treating severe TR that mimic the classical edge-to-edge (Alfieri) surgical repair (Figure 1). The first is the MitraClip System (Abbott Vascular, Santa Clara, CA, USA), a percutaneous edge-to-edge device initially designed for transcatheter mitral valve repair [58–60]. The MitraClip System comprises a steerable guide catheter and a clip delivery system. The delivery system includes a steerable sleeve, a delivery catheter, and a chrome-cobalt clip with two articulated arms to grasp the leaflets. Transcatheter therapy with the MitraClip System has been demonstrated to be effective and durable as an alternative treatment for patients with primary or secondary severe mitral regurgitation. The first experience with transcatheter repair of TR with the MitraClip NT was reported in 2016 [59, 60]. Subsequently, the third-generation MitraClip NTR and XTR were developed in 2018; both have a width of 4 mm, and their arm lengths are 9 mm and 12 mm, respectively. In 2019, the fourth-generation MitraClip G4 was developed, thus expanding the range of clip sizes to a width of 6 mm. This generation also introduced an independent leaflet grasping feature and an updated catheter integrated with a pressure monitor. The MitraClip System, originally designed for mitral valve repair, has since been adapted for TV repair.
After the promising clinical results achieved with the MitraClip for TR treatment, the TriClip Transcatheter TV Repair System (NT and XT) was developed in 2020, on the basis of modification of the MitraClip [61]. The TriClip XT notably offers a 40% larger coaptation surface area than that of the TriClip NT. In 2021, the new-generation TriClip NTW and XTW, featuring wider arms and independent leaflet grasping capabilities, became available.
Another percutaneous edge-to-edge repair device for transcatheter mitral valve repair, the PASCAL System (Edwards Lifesciences, Irvine, CA, USA), was adapted for T-TEER in 2017 [62]. The PASCAL System is composed of a steerable guide sheath, steerable catheter, and implant catheter [63]. The implant itself consists of a central spacer with two adjacent and contoured wide paddles. The central nitinol woven spacer, measuring 10 mm, functions as a filler in the regurgitant orifice and can attach to the leaflets with two spring-loaded paddles, each with a 25 mm width in the grasping position, and clasps measuring 10 mm in length [64]. These clasps are wider than those in the MitraClip and TriClip systems. The clasps can be operated simultaneously or independently for leaflet grasping. A more recent development is the PASCAL Precision System, which offers more precise implant control, an improved handle design, a covered release mechanism, and predictable implant release. This updated version of the PASCAL device is available in two implant sizes. The PASCAL System features broader contoured paddles with a 5 mm spacer, 10 mm width, and a 26 mm wingspan. The PASCAL Ace System has a narrower profile with a 2 mm spacer, 6 mm width, and 26 mm wingspan. Both systems incorporate central spacers to further optimize patient treatment.
Procedural Steps of T-TEER
The main procedural steps of T-TEER are similar between the MitraClip/TriClip System and the PASCAL System. This procedure is conducted in patients under general anesthesia with 2D and 3D TEE and fluoroscopic guidance [65]. The procedure begins with percutaneous venous access obtained through the jugular or femoral vein to introduce a 24-French guide sheath. Unfractionated heparin is administered after the guide sheath enters, with an aim of reaching an activated clotting time of 250–350 seconds throughout the procedure. After the guide sheath enters the RA (Figure 2A), the position of the system is observed with 2D and 3D TEE in bicaval view. Subsequently, the guide sheath is retracted to expose the clip, and the system is bent to orient the clip toward the valve annular plane (Figure 2B). During this process, 2D and 3D TEE are used to correct the clip position and orientation toward the tricuspid commissures in the trangastric short axis view and 3D view. After adjustment of the alignment of the system and trajectory testing, the clip is further advanced into the RV and grasps the leaflets (Figure 2C and 2D). The process of leaflet grasping has been documented with mid- to deep-transesophageal four-chamber views corresponding to long-axis and transgastric views. The residual TR grade and transvalvular gradient are evaluated after release of the first clip (Figure 3B). If a second clip must be implanted, its model and location should be determined according to the current anatomy and the model of the first clip (Figure 2E). Postoperative evaluation should be performed after the clips are implanted (Figure 3C). Finally, the system is retracted, and the puncture site is closed with a “Z-suture” (Figure 2F). Procedural success is defined as safe clip implantation without partial leaflet detachment or device migration and a TR decrease by one or more grades without creation of tricuspid stenosis; an acceptable gradient after clipping has been determined to be ≤3 mmHg [66, 67].
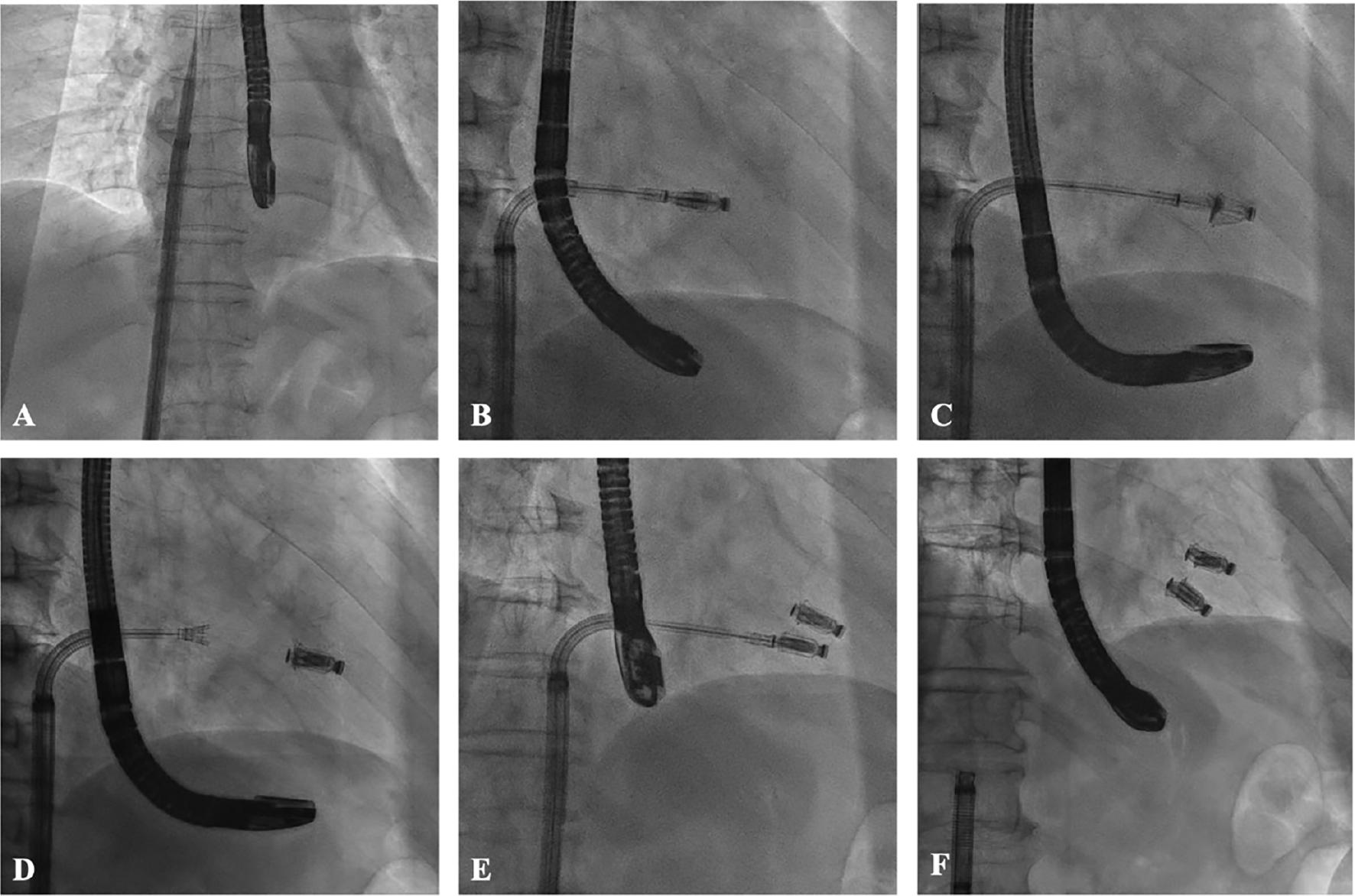
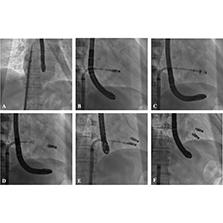
Procedural Steps of Transcatheter Tricuspid Valve Edge-to-Edge Repair with the Neoblazar™ System.
(A) Guide sheath entering the right atrium. (B) The system is bent to orient the clip toward the valve annular plane. (C) The clip enters the right ventricle and grasps the leaflets. (D) Release of the first clip. (E) I Implantation of the second clip. (F) Retraction of the system after the procedure.

Preoperative and Intraoperative Evaluation with Transesophageal Echocardiography during the Transcatheter Tricuspid Valve Edge-to-Edge Repair Procedure.
(A) Preoperative evaluation by transesophageal echocardiography. (B) Evaluation after release of the clip by three-dimensional transesophageal echocardiography. (C) Postoperative evaluation by transesophageal echocardiography after clip implantation.
Current Practice Regarding T-TEER
Clinical Studies with the MitraClip System
The MitraClip System, the most widely used transcatheter TV repair device, is supported by substantial clinical evidence. A notable study by Nikening et al. in 2017 reported 64 patients with chronic, severe TR who underwent MitraClip treatment [68]. In that patient cohort, 88% experienced functional TR, and 34% received combined therapy for severe mitral regurgitation with the MitraClip System. The MitraClip device was successfully implanted in the TV in 97% of patients during the procedure. After treatment, TR decreased by at least one grade in 91% of cases. Importantly, despite this decrease, TR remained severe in 13% of patients. Notably, this study revealed significant decreases in the effective regurgitant orifice area, vena contracta width, and regurgitant volume after the procedure. Although no major procedural complications occurred during the follow-up period, in-hospital deaths occurred in 5% of patients. After a mean follow-up of 14±18 days (ranging from 7 to 45 days), both the New York Heart Association (NYHA) functional class and six-minute walk distance (6MWD) showed significant improvements.
Orban et al. [69] have conducted an analysis of 50 patients with severe TR and right-sided heart failure. After treatment with the MitraClip device over 6 months, significant improvements were observed. Specifically, a TR decrease by at least one grade was achieved in 90% of cases. Moreover, a 79% improvement in NYHA functional class was observed among the patients. Additional notable improvements included a 44% increase in 6MWD, a 30% decrease in median N-terminal pro-B-type natriuretic peptide, and a 16% increase in quality of life scores.
The TriValve registry included 249 patients with severe TR who received treatment with a MitraClip device (NT or NTR/XTR) at 14 sites across Europe and North America between June 2015 and June 2018 [46]. Notably, 52% of these patients also underwent MitraClip treatment for mitral regurgitation as a concurrent therapy. Results from the 1-year follow-up indicated that 77% of patients achieved a TR decrease to a grade of ≤2+ after the successful procedure. Over the 1-year follow-up, 72% of patients maintained a TR grade of ≤2+, and 69% achieved a NYHA functional class of ≤2+. The study identified several predictors of procedural failure, including effective regurgitant orifice area, tricuspid coaptation gap, tricuspid tenting area, and the absence of a central or anteroseptal TR jet location. Additional, predictors of 1-year mortality included procedural failure, worsening kidney function, and the absence of sinus rhythm. This in-depth analysis has offered valuable insights into selecting suitable patients likely to benefit most from this therapeutic approach. The findings from the TriValve registry study provide strong evidence that T-TEER for severe TR is a safe, feasible, and effective method for decreasing TR at the 1-year follow-up, thus ultimately improving clinical outcomes. Further analysis of the TriValve registry study has indicated a decreased diameter of TV annulus after T-TEER, particularly in patients with T-TEER in antero-septal commissure [70].
In a study comparing the MitraClip NTR and XTR systems for transcatheter TV repair, procedural success was achieved in 70% patients with severe TR who were treated with the MitraClip NTR and 80% of patients treated with the MitraClip XTR system [71]. Notably, single-leaflet device attachment (SLDA) was observed in 5% of cases in both groups at the 30-day follow-up. Both groups also experienced an improvement in NYHA functional class. However, the MitraClip XTR system was associated with a greater decrease in TR grade and more effectively decreased torrential TR, owing to its ability to treat larger coaptation gaps.
Another study has reported a 100% technical success rate of the MitraClip XTR system in patients with significant and symptomatic TR; SLDA occurred in 6% of patients after 30 days [72]. Patients treated with the MitraClip XTR system showed decreases in TR grade and NYHA functional class, and an increase in 6MWD. These findings highlight the potential advantages of the MitraClip XTR system for treating symptomatic severe TR.
Clinical Studies with the TriClip System
The TRILUMINATE trial enrolled 85 patients with moderate or greater TR who were treated with the TriClip system between August 1, 2017 and November 29, 2018 [73]. The 6-month results revealed that 86% of patients experienced a decrease of at least one TR grade at 30 days. During the 6-month follow-up, only 4% of patients experienced major adverse events (MAE), and 7% experienced SLDA. All-cause mortality was reported in 5% of patients, and no periprocedural deaths, conversions to surgery, device embolizations, myocardial infarctions, or strokes occurred during this period. The residual TR remained moderate or less in 71% of the participants at 1 year [74], and was sustained in 60% of the participants at 2 years [75]. Furthermore, substantial clinical improvements in NYHA functional class, 6MWD, and the Kansas City Cardiomyopathy Questionnaire (KCCQ) score persisted from 30 days to 2 years. Notably, favorable reverse right ventricular remodeling was observed over the 2-year period. This study thus demonstrated the effectiveness of the TriClip system in decreasing TR in high-risk surgical patients with severe TR and achieving clinical improvement. Further analysis of TRILUMINATE trial data has clarified the improvement in health status after T-TEER, which was associated with a diminished risk of mortality and heart failure hospitalization at 1 year [76]. However, the potential generalizability of the TRILUMINATE eligibility criteria to real-world conditions merits further research [77].
A Spanish multicenter study conducted between June 2020 and March 2021 has found that all the 34 enrolled patients experienced a TR decrease by at least one grade [78]. At discharge, the study observed a TR severity of ≤2 in 91% of patients, and this level was maintained in 80% of the patients at the 3-month follow-up.
The bRIGHT post-approval study was a prospective, single-arm, open-label, multicenter, postmarket registry [79]. This real-world observational study enrolled 511 participants with symptomatic severe TR across 26 centers in Europe. The results at 30 days showed that the TriClip device was successfully implanted in 99% of the patients, and TR was decreased to moderate or less in 77% of patients within that period. Improvements in NYHA functional class and KCCQ score were observed at 30 days. Importantly, during the 30-day follow-up, only 2.5% of participants experienced a MAE.
The TRILUMINATE Pivotal study, a recent prospective randomized trial, was conducted at 65 sites in the United States, Canada, and Europe between August 21, 2019, and September 29, 2021 [80]. The study enrolled 350 eligible patients with symptomatic severe TR. The 1-year results indicated a TR decrease to moderate or less in 87.0% of patients in the T-TEER group and only 4.8% of patients in the control group. Additionally, the KCCQ score showed greater improvements in the T-TEER group than the control group at 30 days. The rate of MAEs was relatively low, occurring in only 1.7% of participants during the 30-day follow-up.
Clinical Studies with the PASCAL System
In a multicenter observational study, 28 high-risk surgical patients with symptomatic severe TR were treated with the PASCAL System between September 2017 and February 2019 at six centers [81]. Most patients (92%) had functional TR. The procedure was successful in 86% of participants. During the 30-day follow-up period, SLDA was observed in 7% of patients. After the procedure, 88% of patients improved to NYHA functional class I/II, and 85% had a TR grade ≤2. Additionally, the 6MWD improved with respect to the baseline at 30 days.
In 2021, the 30-day outcomes from the ongoing U.S. prospective, multicenter, single-arm CLASP TR early feasibility study were reported [82]. The PASCAL System was successfully implanted in 85% of the 34 patients in the study. At 30 days, 85% of patients achieved a decrease by at least one TR grade, and 52% had TR of moderate or less. A minor percentage (5.9%) of participants experienced MAEs, and no instances of cardiovascular mortality, stroke, myocardial infarction, renal complications, or reintervention were observed. Notably, 89% of the patients improved to NYHA functional class I/II, and both the 6MWD and the KCCQ score increased at 30 days.
In the recent 1-year results of the CLASP TR study [83], 65 patients with symptomatic severe functional or degenerative TR who were treated with the PASCAL System were analyzed. After 1 year, TR severity remained moderate or less in 86% of patients, and 100% had at least a one-grade decrease in TR. During the 1-year follow-up, the rate of freedom from all-cause mortality was 87.9%, and the rate of freedom from heart failure hospitalization was 78.5%. Additionally, 92% of patients improved to NYHA functional class I/II. The 6MWD and the KCCQ scores remained improved between 30 days and 1 year, thereby indicating sustained clinical benefits.
Aurich et al., in their initial experience with the PASCAL Ace System in patients with severe TR [84], achieved procedural success in 75% of 16 participants. A decrease in TR was observed in 69% of patients. Additionally, a significant improvement in NYHA functional class was observed in 73% of patients who experienced decreased TR at 1 week. Importantly, the study also noted a significant decrease in right atrial volume and right ventricular end-diastolic diameter after the procedure. These findings indicate that the PASCAL Ace System may not only decrease TR but also improve right ventricular remodeling.
The PASTE registry study, a retrospective, multicenter, observational cohort study conducted at eight European sites in Germany and Switzerland, included 235 high-risk surgical patients with symptomatic severe TR [85]. The PASCAL or PASCAL Ace system was successfully implanted in 78% of patients. A decrease in TR to moderate or less was observed in 78% of patients. At the median follow-up period of 173 days, a TR decrease to moderate or less was sustained in 78% of patients. Furthermore, right ventricular remodeling was observed during the follow-up. Approximately 63% of patients improved to NYHA functional class I/II at follow-up. Importantly, no significant difference in TR decrease was observed between the PASCAL and PASCAL Ace systems, thus indicating their similar effectiveness in decreasing TR.
The TriCLASP study was a prospective, single-arm, multicenter study designed to assess the safety and performance of the PASCAL system [86]. The 30-day results of the TriCLASP study indicated a significant decrease in TR severity that was observed at discharge and was sustained at 30 days. Notably, approximately 90.0% of patients achieved TR of moderate severity or less. The composite rate of MAE at 30 days was only 3.0%, thus demonstrating the safety of the procedure. No nonelective TV reinterventions, major access site and vascular complications, major cardiac structural complications, or device embolizations were observed during the follow-up period. Additionally, the patients showed improvements in NYHA class, 6MWD, and KCCQ scores at 30 days. These results highlight the efficacy and safety of the PASCAL system in treating TR.
Prognostic Factors after T-TEER
Beyond the therapeutic effects of T-TEER, factors associated with poor outcome have attracted increasing attention. Bartkowiak et al. have used deep learning detection techniques for anatomical computed tomography in patients who underwent T-TEER. The computed tomography-derived RV length and height between the inferior vena cava and TV annulus were associated with elevated risk of procedural complexity and adverse outcomes [87]. Right ventricular cardiac power index (RVCPi), a hemodynamic parameter of RV performance combining the cardiac index with the mean pulmonary artery pressure, was associated with 1-year all-cause mortality and heart failure hospitalization in patients with severe TR after T-TEER [88]. This study suggests that RVCPi has merit for the prognostication and risk stratification of T-TEER candidates. In addition, increase in RV fractional area change after the T-TEER procedure was associated with decreased mortality and heart failure hospitalization in patients with an RV fractional area change <35% within 1 year [89]. Retrospective analysis of the TriValve registry study has suggested that a post-procedural increase in the TV gradient is irrelevant to 1-year all-cause mortality and heart failure hospitalization [90]. Another study has indicated that higher estimated stressed blood volume is an independent predictor of death and heart failure hospitalization in patients with severe TR receiving T-TEER [91]. Vogelhuber et al. have investigated the effects of different body mass index categories on clinical outcomes after T-TEER, and have confirmed underweight and obesity as independent risk factors for 1-year all-cause mortality [92]. Further investigations are needed to achieve optimal intraprocedural decision-making in patients with differing risk stratification.
Future Directions
T-TEER has emerged as a safe and effective procedure for patients with symptomatic severe TR if anatomy is favorable, but several challenges remain. One major challenge is selecting potentially suitable patients who will benefit the most from T-TEER. The future of the T-TEER procedure should involve personalized risk assessment and more refined patient selection based on clinical, hemodynamic, and anatomical factors. The complex anatomy of the TV apparatus should be considered during the procedure. Rare but potentially serious complications, such as SLDA, can occur as a result of leaflet insertion loss within the clip or torn leaflet tissue. To minimize the risk of post-procedural complications, optimized device design and innovative repair approaches are required. Additional challenges arise from relying solely on TEE imaging during the procedure. Problems such as severe right heart enlargement, a horizontal heart axis, and acoustic shadowing from left-sided prosthetic valves or device catheters can complicate imaging. Moreover, 3D intracardiac echocardiography (ICE) has an emerging role for patients with contraindications to TEE or insufficient imaging quality [93]. Combining 3D ICE with TEE during procedures may help overcome these imaging challenges [94, 95]. As the technology improves, 3D ICE may gradually replace TEE as the imaging modality of choice for T-TEER.
The TriClip and PASCAL System have already received European CE-mark approval for the treatment of TR. These devices have not currently been approved for treating TR by the U.S. Food and Drug Administration. In China, several innovative T-TEER devices have been developed and have entered clinical trials in recent years. The Neoblazar system is a T-TEER device independently designed and developed in China. The first-in-human experience with the Neoblazar™ System (Dawneo Medical Technology, Hangzhou, China) demonstrated high procedural success, and supported the feasibility and safety of the new system in patients with severe TR (unpublished). The NoTR study (NCT05497141), a prospective, multicenter, single arm, objective performance criteria trial conducted at our hospital and other sites, has completed the patient recruitment according to the inclusion/exclusion criteria. The novel DragonFly-T™ Tricuspid Repair system (Valgen Medical, Hong Kong, China) is under clinical investigation (NCT05556460 and NCT05671640). Additionally, the K-Clip™ transcatheter tricuspid annuloplasty system (Huihe Medical Technology, Shanghai, China) has recently been used successfully for tricuspid intervention [96, 97]. Further results from our study and others (Table 3) involving larger patient populations and longer clinical follow-up periods will be necessary to confirm the efficacy and durability of T-TEER, and to contribute to the ongoing improvement and refinement of the procedure.
Ongoing Studies on Transcatheter Tricuspid Valve Edge-to-Edge Repair for Treating Severe Tricuspid Regurgitation.
| Study | Device | Study design | Study start (actual) | Study completion (estimated) | Sample size | Primary endpoints |
|---|---|---|---|---|---|---|
| CLASP TR EFS | PASCAL | Prospective, single arm | 2019-02-05 | 2026-05-31 | 65 | Freedom from device or procedure-related adverse events at 30 days |
| TRILUMINATE Pivotal | TriClip | Prospective randomized controlled | 2019-08-21 | 2028-12 | 1500 | Hierarchical composite of number of participants with all-cause mortality or number of participants with TV surgery, rate of heart failure hospitalizations, and assessment of quality of life improvement (KCCQ score) at 12 months |
| CLASP II TR | PASCAL | Prospective randomized controlled | 2019-12-11 | 2029-03-31 | 870 | Composite endpoint including all-cause mortality, right ventricular assist device implantation or heart transplant, TV intervention, heart failure hospitalizations, and quality of life improvement (KCCQ score) at 24 months |
| bRIGHT EU PAS | TriClip | Prospective, single arm | 2020-08-27 | 2028-01 | 501 | Acute procedural success up to 30 days (TR decrease by at least 1 grade at discharge; 30-day echocardiogram will be used if discharge is unavailable or uninterpretable) |
| German Registry | TriClip, PASCAL | Prospective, single arm | 2020-10-01 | 2030-12-31 | 600 | All-cause death and heart failure hospitalization at 12 months |
| Pforzheim TV Registry | TriClip | Prospective, single arm | 2020-11-30 | 2026-11-30 | 200 | Procedural success at 12 months (one-grade TR decrease) |
| TriCLASP | PASCAL | Prospective, single arm | 2021-01-15 | 2028-03-15 | 300 | MAE rates at 30 days; change in TR severity at discharge or 7 days post-procedure |
| TRI-FR | TriClip | Prospective randomized controlled | 2021-02-10 | 2024-03 | 300 | Milton Packer clinical composite score at 12 months* |
| PASTE | PASCAL | Retro- and prospective, single arm | 2021-05-05 | 2024-05-30 | 1000 | Change in TR severity (TR≤ moderate) at 12 months |
| TRICI-HF | TriClip, PASCAL | Prospective randomized controlled | 2022-02-25 | 2027-03-01 | 360 | All-cause mortality or heart failure hospitalization at 12 months |
| NoTR | Neoblazar | Prospective, single arm | 2022-07-22 | 2023-10-22 | 98 | Rate of implantation success at 12 months (no all-cause mortality after implantation; no tricuspid open surgery after implantation; TR decrease by at least 1 grade) |
| DragonFly-T | DragonFly-T | Prospective, single arm | 2022-10-15 | 2028-10-15 | 151 | All-cause death and recurrent heart failure hospitalization at 12 months |
| DragonFly-T | DragonFly-T | Prospective, single arm | 2022-10-27 | 2024-01-10 | 10 | Incidence of MAE at 30 days |
| TRACE-NL | TriClip, PASCAL | Prospective randomized controlled | 2022-12-12 | 2027-11-01 | 150 | Hierarchical composite of all-cause mortality, heart failure hospitalization, and KCCG score at 12 months |
| TriBEL | Not mentioned | Retro- and prospective, single arm | 2023-01-01 | 2029-12-31 | 120 | TR decrease by at least one grade at 30 days; composite of MAE at 1 year |
*The Milton Packer clinical composite score classifies each patient into one of three categories (improved, worsened, or unchanged), and is determined aggregating evaluation by using New York Heart Association functional classification, quality of life score using patient global assessment, and number of major cardiovascular events.
TV: tricuspid valve; KCCG: Kansas City Cardiomyopathy Questionnaire; TR: tricuspid regurgitation; MAE: major adverse event.
Conclusion
TV, often considered a “forgotten valve,” has become a growing focus in the field of interventional surgery. The development of interventional procedures such as T-TEER has led to less invasive and safer treatment approaches for TR. Preliminary results from T-TEER devices have shown great promise. As the understanding of the TV continues to improve, and transcatheter treatments progress, T-TEER may become a preferred method for managing symptomatic severe TR in high-risk surgical patients. Because anatomical limitations still limit patient eligibility, technological innovation and clinical research are required to expand patient indications. Interpretation of echocardiographic, cardiac computed tomography, and cardiac magnetic resonance images should be explored in greater depth. Future work will be key to determining the place of T-TEER in the treatment landscape for this condition.