INTRODUCTION
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to more than 575 million confirmed cases and 6 million deaths worldwide through August 2, 2022 [1]. Efficacious vaccines are important preventive measures against COVID-19. According to World Health Organization (WHO) data released on June 1, 2022, more than 300 COVID-19 vaccine candidates have been developed or are in development. Of these, 153 COVID-19 vaccine candidates have been evaluated in clinical trials. These vaccines mainly include inactivated vaccines (accounting for 14% of the total), live attenuated vaccines (1%), viral vector vaccines (replication and non-replication; 17% of the total), RNA vaccines (18%), DNA vaccines (11%), protein subunit vaccines (34%), and VLP vaccines (4%) [2].
The first generation COVID-19 vaccines were designed on the basis of the receptor-binding domain (RBD) or spike protein of the prototype SARS-CoV-2 or the whole prototype virus. The WHO Emergency Use Listing (EUL) has authorized 11 vaccines for emergency use through June 13, 2022, including Ad26.COV2.S developed by Janssen, COVAXIN developed by Bharat Biotech, BNT162b2 developed by Pfizer-BioNTech, AZD1222 Vaxzevria developed by Oxford-AstraZeneca, mRNA-1273 developed by Moderna, inactivated SARS-CoV-2 Vaccine BBIBP-CorV developed by Sinopharm, CoronaVac developed by Sinovac, NVX-CoV2373/Nuvaxovid developed by Novavax, ChAdOx1 nCoV-19 and NVX-CoV2373/Covovax developed by the Serum Institute of India, and Ad5-nCoV developed by CanSino Biologics [3]. These vaccines on the WHO EUL, along with seven other vaccine products conditionally approved by national regulatory authorities, are already in large-scale use [3,4] to mitigate the SARS-CoV-2 pandemic by preventing severe illness or death, and inducing herd immunity in populations.
All populations are susceptible to SARS-CoV-2 infection, including children, adults, older people, males and females, and people with underlying medical conditions. Some populations have relatively higher risk of severe COVID-19 or severe outcomes of SARS-CoV-2 infection [5]. The large-scale vaccination campaigns worldwide have inevitably led to increasing studies on the use of COVID-19 vaccines in special populations with underlying medical conditions, such as people with cancer, pregnancy, rheumatic and/or musculoskeletal diseases, long-term hemodialysis, or solid organ transplantation [6,7]. Vaccine protection against COVID-19 may differ in these populations.
In this review, we systematically summarize the efficacy and/or effectiveness of first generation COVID-19 vaccines in clinical trials or real-world studies against the prototype strain or various SARS-CoV-2 variants, in both generally healthy populations and populations with underlying conditions or diseases. In addition, we compare the enhanced efficacy and/or effectiveness associated with various boosting immunization strategies with COVID-19 vaccines. The gaps in understanding of the persistence and spectrum of vaccine protection conferred by currently available COVID-19 vaccines are also discussed.
METHODS
We searched PubMed with the key terms “(COVID-19[Title/Abstract] OR SARS-CoV-2[Title/Abstract]) AND (vaccine[Title/Abstract] OR vaccination[Title/Abstract]) AND (efficacy[Title] OR effectiveness[Title/Abstract] OR protection[Title/Abstract] OR effect[title/Abstract])” and “(protection [Title/Abstract]) AND ((SARS-CoV-2 [Title/Abstract]) OR (COVID-19 vaccination[Title/Abstract]) OR (breakthrough infection[Title/Abstract])),” with article types restricted to “clinical study,” “clinical trial,” or “observational study.” A total of 319 and 161 articles were found with these searches, respectively. Duplicated studies and meta-analyses were excluded. Additional publications were identified through manual searching of the reference sections of each article identified with the above keywords. We identified a total of 99 articles reporting phase 3 trials or real-world studies determining the efficacy or effectiveness of COVID-19 vaccines, published before July 20, 2022, by reading the titles and abstracts.
COVID-19 VACCINES ON THE WHO EUL
Inactivated vaccines
Three inactivated COVID-19 vaccines are included on the WHO EUL: BBIBP-CorV and CoronaVac developed in China, which are β-propiolactone inactivated prototype vaccines adjuvanted with aluminum hydroxide [8], and BBV152 developed in India, which is also a β-propiolactone-inactivated whole-virion SARS-CoV-2 vaccine but is adjuvanted with Algel-IMDG, an imidazoquinoline class molecule (TLR7 and TLR8 agonist) adsorbed onto Algel [9].
The first phase 3 efficacy trial of CoronaVac, performed between July 2020 and January 2021 in Turkey, Brazil and Indonesia, reported an efficacy of 83.5% (95% CI 65.4–92.1), 50.7% (95% CI, 36.0–62.0), and 65.3% (not available for 95% CI) in preventing PCR-confirmed symptomatic COVID-19 at 14 or more days after the second dose, respectively (Table 1) [10–12].
Efficacy of COVID-19 vaccines in phase 3 randomized controlled clinical trials.
| Sponsor/country | Vaccine | Dose/regimen | Region/country | Study period | VE (any variant) | VE against COVID-19 of any severity (any variant) | VE in the older population | VE against VOC | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild/moderate | Severe/critical | Alpha (B.1.1.7) | Beta (B.1.351) | Gamma (P.1) | Delta (B.1.617.2) | |||||||
| Sinovac/China | CoronaVac | 2 doses/14 days apart | Turkey [10] | September 2020 to January 2021 | 83.5% (65.4–92.1) | - | - | - | - | - | - | - |
| Brazil [11] | July to December 2020 | 50.7% (36.0–62.0) | 100% (56.4–100.0)* | 100% (16.9–100.0) | 51.1% (−166.9–91.0)† | |||||||
| Indonesia [12] | August to October 2020 | 65.30% | - | - | ||||||||
| Beijing CNBG/China | BBIBP-CorV | 2 doses/21 days apart | United Arab Emirates and Bahrain [13] | July to December 2020 | 78.1% (64.8–86.3) | - | 100% | - | - | - | - | - |
| Bharat Biotech/India | BBV152 | 2 doses/28 days apart | India [14] | November 2020 to January 2021 | 77.8% (65.2–86.4) | - | 93.4% (57.1–99.8) | 67.8% (8.0–90.0)† | - | - | - | 65.2% (33.1–83.0) |
| AstraZeneca/United Kingdom | AZD1222 | 2 doses/21–35 days apart | United Kingdom and Brazil [15] | April to November 2020 | 62.1% (41.0–75.7) | - | - | - | - | - | - | - |
| United States, Chile, and Peru [16] | - | 74.0% (65.3–80.5) | - | 100% | 83.5% (54.2–94.1)‡ | - | - | - | ||||
| South Africa [17] | June to December 2020 | - | 21.9% (−49.9–59.8) | - | - | - | 10.4% (−76.8–54.8) | - | ||||
| United Kingdom [18] | May to November 2020 | - | - | - | - | 70.4% (43.6–84.5) | - | - | ||||
| Brazil [19] | June to December 2020 | - | - | - | - | - | - | 64% (−2.1–87.0) | ||||
| Janssen/United States | Ad26.COV2.S | 1 dose | Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, and United States | September 2020 to January 2021 [20] | 66.9% (59.1–73.4) | 64.8% (55.8–72.2) | 76.7% (54.6–89.1) | 76.3% (61.6–86.0)† | - | - | - | - |
| September 2020 to July 2021 [21] | 55.9% (51.0–60.5) | 29.4% (−64.6–70.7) 52.1% (46.1–57.4) | 73.3% (63.9–80.5) | 55.0% (42.9–64.7)† | 70.1% (35.1–87.6) | 38.1% (4.2–60.4) | 36.4% (13.9–53.2) | −6.0% (−178.3–59.2) | ||||
| CanSino/China | Convidecia | 1 dose | Argentina, Chile, Mexico, Pakistan, and Russia [22] | September 2020 to January 2021 | 57.5% (39.7–70.0) | - | - | 17.5% (−127.6–70.1)† | - | - | - | - |
| Novavax/United States | NVX-CoV2373 | 2 doses/21 days apart | United Kingdom [23] | September to November 2020 | 89.7% (80.2–94.6) | - | - | 88.9% (20.2–99.7)‡ | 86.3% (71.3–93.5) | - | - | - |
| United States and Mexico [24] | December 2020 to February 2021 | 90.4% (82.9–94.6) | - | 100% (87.0–100) | - | 92.6% (83.6–96.7) | ||||||
| Pfizer/BioNTech/United States/Germany | BNT162b2 | 2 doses/21 days apart | United States, Argentina, Brazil, South Africa, Germany, and Turkey | July to November 2020 [25] | 95.0% (90.3–97.6) | - | 75.0% (−152.6–99.5) | 94.7% (66.7–99.9)‡ | - | - | - | - |
| July to October 2020 [26] | 91.3% (89.0–93.2) | 96.7% (80.3–99.9) | 94.5% (88.3–97.8)‡
96.2% (76.9–99.9)|| | 100% (53.5–100) | ||||||||
| Moderna/United States | mRNA-1273 | 2 doses/28 days apart | United States [27] | July to October 2020 | 94.1% (89.3–96.8) | - | 100% | 86.4% (61.4–95.2)‡ | - | - | - | - |
| United States [28] | July to October 2020 | 93.2% (91.0–94.8) | 98.2% (92.8–99.6) | 91.5% (83.2–95.7)‡ | - | |||||||
| United States and Canada [29] | March to August 2021 | - | - | - | 88.0% (70.0–95.8)‡‡ | |||||||
| Gamaleya/Russia | Sputnik V | 2 doses/21 days apart | Russia [30] | September to November 2020 | 91.6% (85.6–95.2) | - | 100% (94.4–100.0) | 91.8% (67.1–98.3)§ | - | - | - | - |
| Wuhan CNBG/China | WIBP-CorV | 2 doses/21 days apart | United Arab Emirates and Bahrain [13] | July to December 2020 | 72.8% (58.1–82.4) | - | 100% | - | - | - | - | - |
| Zhifei Longcom/China | ZF2001 | 3 doses/30 days apart | Uzbekistan, Indonesia, Pakistan and Ecuador [31] | December 2020 to December 2021 | 75.7% (71.0–79.8) | - | 87.6% (70.6–95.7) | 67.6% (21.9–87.8)† | 88.3% (66.8–97.0) | - | - | 76.1% (70.0–81.2) |
| Clover Biopharmaceuticals/Hong Kong, China | SCB-2019 | 2 doses/21 days apart | Belgium, Brazil, Colombia, Philippines and South Africa [32] | March to August 2021 | 67.2% (54.3–76.8)¶ | 83.7% (55.9–95.4)* | 100% (25.3–100.0)** | 58.4% (−73.4–92.9)† | - | - | 91.8% (44.9–99.8) | 78.7% (57.3–90.4) |
| GSK+Medicago/United States and Canada | CoVLP+AS03 | 2 doses/21 days apart | Argentina, Brazil, Canada, Mexico, United Kingdom, and United States [33] | March to September 2021 | 69.5% (56.7–78.8) | 76.9% (51.5–90.0) 78.8% (55.8–90.8)* | 100.0% (−63.7−NA) | 12.9% (−3295.5–97.8)‡ | 100% (38.2–NA) | - | 87.8% (73.0–95.3) | 74.05% (51.7–86.8) |
| Cadila Healthcare/India | ZyCoV-D | 3 doses/28 days apart | India [34] | January to June 2021 | 66.6% (47.6–80.7) | 64.9% (44.9–79.8) | 100% | - | ||||
| CureVac/Germany | CVnCoV | 2 doses/28 days apart | Europe and Latin America [35] | December 2020 to April 2021 | 48.2% (31.0–61.4)†† | 70.7% (42.5–86.1)* | - | - | 55.1% (23.5–73.6) | - | 67.1% (29.8–84.6) | - |
VE (vaccine efficacy) is represented with point estimates and their 95% confidence intervals.
*Represents moderate to severe disease.
†Represents the older population ≥60 years of age.
‡Represents the older population ≥65 years of age.
§Represents the older population >60 years of age.
||Represents the older population ≥75 years of age.
¶Represents 95.72% CI.
**Represents 97.86% CI.
††Represents 95.826% CI.
‡‡Represents children 6–11 years of age receiving one dose of mRNA-1273.
From January 2021 to February 2022, five retrospective or prospective cohort studies or test-negative case-control studies on CoronaVac reported moderate or high vaccine effectiveness against SARS-CoV-2 variant associated symptomatic infection, hospitalization, or death (Table 2). A countrywide mass vaccination campaign with CoronaVac was conducted in Chile from February to May 2021, with the predominant strain of Gamma (P.1). The vaccine showed an effectiveness of 65.9% (95% CI, 65.2–66.6) against COVID-19, 87.5% (95% CI, 86.7–88.2) against hospitalization, and 86.3% (95% CI, 84.5–87.9) against COVID-19-associated death [36]. However, the effectiveness of CoronaVac in later studies was comparable to or slightly lower than those values in settings involving new epidemic variant transmission or older populations. A retrospective analysis involving all confirmed cases of COVID-19 in mainland China between May 21, 2021 and February 28, 2022 was conducted. In that period, the epidemic variants shifted from Delta (B.1.617.2) to Omicron (B.1.1.529) [75], thus demonstrating that full vaccination decreased the risk of severe COVID-19 disease by 83% and 69%, and booster vaccination decreased the risk of pneumonia by 86% and 69%, and the risk of severe disease by 98% and 91%, among people 18–59 and ≥60 years of age, respectively. Another study in Hong Kong reported that primary two-dose immunization of CoronaVac conferred high protection (approximately 91.7%–93.3%) against severe, critical disease, and death in adults ≤59 years of age, but the protection was lower (approximately 58.2%–63.0%) in adults 60 years or older, particularly those older than 80 years of age [76].
Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants in real-world studies.
| Sponsor/country | Name of vaccine | Study design | Country/countries | Study period | Effectiveness against SARS-CoV-2 variants | |
|---|---|---|---|---|---|---|
| Sinovac/China | CoronaVac [36–40] | 2 retrospective cohort studies; 2 prospective cohort studies; 1 test-negative case-control study | Chile, China, Turkey, and Brazil | January 2021 to February 2022 |
 |
 |
| Beijing Institute of Biological/China | BBIBP-CorV [40–43] | 2 retrospective cohort studies; 1 test-negative, case-control study; 1 retrospective, observational study | China, Argentina, Hungary, and the United Arab Emirates | September 2020 to September 2021 |
 | |
| Bharat Biotech/India | BBV152 [44] | 1 test-negative, case-control study | India | April 2021 to May 2021 |
 | |
| AZD1222/United Kingdom | AZD1222 Vaxzevria [41,43,45–50] | 6 test-negative case-control studies; 1 large community-based survey; 1 retrospective, observational study | United Kingdom, Scotland, Canada, Brazil, Argentina, and Hungary | December 2020 to January 2022 |
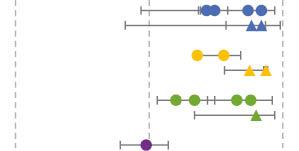 | |
| Janssen/United States | Ad26.COV2.S [51,52] | 1 test-negative design study; 1 matched cohort design study | South Africa | November 2021 to January 2022 |
 | |
| CanSino/China | Convidecia [53] | 1 retrospective cohort study | China | In July 2021 |
 | |
| Gamaleya/Russia | Sputnik V [41,43] | 1 test-negative, case-control study; 1 retrospective, observational study; 1 retrospective cohort study | Argentina and Hungary | December 2020 to September 2021 |
 | |
| Pfizer/BioNTech/United States/German | BNT162b2 [43,45,46,48,49,50,52,54–72] | 11 test-negative case-control studies; 6 retrospective cohort studies; 5 prospective cohort studies; 3 observational studies | United States, United Kingdom., Israel, Italy, Qatar, Canada, France, Hungary, Scotland, South Africa, and Germany | February 2020 to February 2022 |
 | |
| Moderna/United States | mRNA-1273 [43,46,48,56,64,65,68,70,72–74] | 6 test-negative case-control studies; 2 retrospective cohort studies; 2 prospective cohort studies; 1 observational study | United States, Qatar, Canada, France, Hungary, and United Kingdom | December 2020 to January 2022 |
 | |
Real-world vaccine effectiveness, as assessed by case-control and observational studies of authorized two-dose regimens for CoronaVac, BBIBP-CorV, BBV152, AZD1222, Sputnik V, BNT162b2, and mRNA-1273, and a one-dose regimen for Ad26.COV2.S and Ad5-nCoV. The point estimates and their 95% confidence intervals reported in studies of different vaccines are shown on the right, with circles representing vaccine effectiveness against infection/symptomatic infection and triangles representing severe disease, including hospitalization or death. Some studies reported vaccine effectiveness only in older adults or children, as indicated.
*Older adults ≥70 years of age.
†Children 3–5 years of age.
‡Older adults ≥60 years of age.
§Older adults 60–79 years of age and after the first component of Sputnik V.
||Older adults ≥80 years of age.
¶Children 5–11 years of age.
**One-dose mRNA-1273 effectiveness.
Generally, CoronaVac has been reported to confer high protection against severe COVID-19, hospitalization, and intensive care unit (ICU) admission among children and adolescents [77]. In children 6–16 years of age, the effectiveness of CoronaVac against infection was 74.5% (95% CI, 73.8–75.2), that against hospitalization was 91.0% (95% CI, 87.8–93.4), and that against ICU admission was 93.8% (95% CI, 87.8–93.4). However, a population-based cohort study of 490,694 children 3–5 years of age, who were observed to evaluate the effectiveness of CoronaVac during the Omicron (B.1.1.529) outbreak in Chile, reported a clear decrease in vaccine protection against Omicron (B.1.1.529) variant infection [38]. The estimated vaccine effectiveness was 38.2% (95% CI, 36.5–39.9) against symptomatic COVID-19, 64.6% (95% CI, 49.6–75.2) against hospitalization, and 69.0% (95% CI, 18.6–88.2) against ICU admission.
Another inactivated COVID-19 vaccine, BBIBP-CorV, manufactured by Beijing Institute of Sinopharm, was developed on the basis of the prototype SARS-CoV-2 HB02 strain. An efficacy study of BBIBP-CorV conducted in the United Arab Emirates and Bahrain among adults 18 years and older without a known history of COVID-19, from July 2020 to December 2020, indicated an efficacy of 78.1% (95% CI, 64.8–86.3) against symptomatic COVID-19 during a median follow-up of 77 days (range: 1–121), starting 14 days after the second dose (Table 1) [13]. Vaccine effectiveness of BBIBP-CorV has been evaluated in China, Argentina, Hungary, and the United Arab Emirates (Table 2). In Argentina, a significantly lower risk of SARS-CoV-2 infection and COVID-19 death in people older than 60 years was reported with BBIBP-CorV vaccination, with a vaccine effectiveness of 44% (95% CI, 42–45) and 85.0% (95% CI, 84.0–86.0), respectively [41]. A real-world effectiveness study conducted in Singapore suggested that BBIBP-CorV is better than CoronaVac in preventing infection and severe disease, but both BBIBP-CorV and CoronaVac confer significantly weaker protection than the mRNA vaccine. On the basis of this evidence, Singapore requires vaccination with three doses of inactivated vaccine as the primary immunization series [78].
In another study, the efficacy and effectiveness of BBV152 were evaluated in India. The efficacy study, involving 25 Indian hospitals or medical clinics, recruited adults (≥18 years of age) who were healthy or had stable chronic medical conditions between November 16, 2020, and January 7, 2021, when the Delta (B.1.617.2) was the predominant pandemic variant [14]. The overall estimated BBV152 vaccine efficacy was 77.8% (95% CI, 65.2–86.4) for symptomatic cases and 93.4% (95% CI, 57.1–99.8) for severe cases (Table 1). The efficacy was 67.8% (95% CI, 8.0–90.0) for older people (≥60 years) and 79.4% (95% CI, 66.0–88.2) for people younger than 60 years. However, the effectiveness data indicated that the protection conferred by BBV152 against symptomatic COVID-19 diseases decreased when Delta (B.1.617.2) became predominant in the pandemic [44].
Viral vector vaccines
The ChAdOx1 nCoV-19 vaccine (AZD1222), developed by Oxford University, consists of a replication-deficient chimpanzee adenoviral vector, ChAdOx1, containing the SARS-CoV-2 structural spike protein gene. In phase 3 trials conducted in the United Kingdom and Brazil, two doses of ChAdOx1 nCoV-19 showed an overall vaccine efficacy of 62.1% (95% CI, 41.0–75.7) in adults 18 years of age and older (Table 1) [15]. Another phase 3 trial in the United States, Chile, and Peru, estimated that two doses of ChAdOx1 nCoV-19 vaccines conferred an overall vaccine efficacy of 74.0% (95% CI, 65.3–80.5) in participants 18 years of age or older and 83.5% (95% CI, 54.2–94.1) in participants 65 years of age or older [16]. However, in a multicenter, double-blind, randomized, controlled trial in South Africa, the two-dose regimen of ChAdOx1 nCoV-19 vaccine demonstrated nearly no protection 10.4% (95% CI, –76.8–54.8) against mild-to-moderate COVID-19 due to the Beta (B.1.351) variant [17]. With the massive administration of the ChAdOx1 nCoV-19 vaccine in populations, the effectiveness of this vaccine has been assessed in the United Kingdom, Scotland, Canada, Brazil, Argentina, and Hungary (Table 2). Surveillance data on symptomatic cases of COVID-19 in England revealed an effectiveness of two doses of the ChAdOx1 nCoV-19 vaccine of 74.5% (95% CI, 68.4–79.4) among people exposed to the Alpha (B.1.1.7) variant and 67.0% (95% CI, 61.3–71.8) among those exposed to the Delta (B.1.617.2) variant [45]. During the Omicron (B.1.1.529) epidemic, two-dose immunization with the ChAdOx1 vaccine induced a protection of approximately 48.9% (95% CI, 39.2–57.1) against symptomatic infections 2–4 weeks after second dose, which subsequently waned over time. Two doses of the ChAdOx1 vaccine conferred little to no protection against Omicron (B.1.1.529)-associated infection 6 months after the second vaccination [46].
The Ad26.COV2.S (Johnson & Johnson) vaccine, developed by Janssen, is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length SARS-CoV-2 spike protein in a prefusion-stabilized conformation. In phase 3 trials, a single dose of Ad26.COV2.S conferred protection of 66.9% (95% CI, 59.1–73.4) and 55.9% (95% CI, 51.0–60.5) against symptomatic COVID-19 of any severity, and 76.7% (95% CI, 54.6–89.1) and 73.7% (95% CI, 63.9–80.5) against severe to critical COVID-19 disease, with an onset of protection at least 14 days after vaccination (Table 1) [20,21]. Ad26.COV2.S conferred significant protection against the Alpha (B.1.1.7), Beta (B.1.351), and Gamma (P.1) variants, but not the Delta (B.1.617.2) variant [21]. The effectiveness of a single dose of the Ad26.COV2.S vaccine, evaluated in South Africa from November 2021 to January 2022 (Table 2), was 75% (95% CI, 69–82) in preventing COVID-19-associated hospital admissions requiring critical or intensive care, and 62% (95% CI, 42–76) and 67% (95% CI, 62–71) in preventing COVID-19-associated hospitalizations [51] during epidemics dominated by the Beta (B.1.351) and Delta (B.1.617.2) variants.
Convidecia is a single-dose Ad5 vectored vaccine expressing the SARS-CoV-2 spike protein (Ad5-nCoV vaccine), manufactured by CanSino Biologics, China. A phase 3 clinical trial enrolling adults 18 years of age and older, performed in Argentina, Chile, Mexico, Pakistan and Russia, found that one dose of Convidecia had a 57.5% (95% CI, 39.7–70.0) efficacy against symptomatic, PCR-confirmed SARS-CoV-2 infection at 28 days or more after vaccination (Table 1) [22]. Only one observational study, in Yunnan province in China, has reported the effectiveness of the Ad5-nCoV vaccine Convidecia [53]; the study indicated a protection of 61.5% (95% CI, 9.5–83.6) against symptomatic COVID-19, 67.9% (95% CI, 1.7–89.9) against COVID-19 pneumonia, and 100% (95% CI, 36.6–100) against severe/critical COVID-19 caused by the Delta (B.1.617.2) variant.
Protein subunit vaccine
NVX-CoV2373 is the only recombinant protein vaccine currently on the WHO EUL. It contains a recombinant nanoparticle prefusion spike protein of the prototype strain plus Matrix-M adjuvant. In a phase 3 trial conducted at 33 sites in the United Kingdom, in adults 18–84 years of age, two doses of NVX-CoV2373 showed an overall vaccine efficacy of 89.7% (95% CI, 80.2–94.6) against symptomatic disease largely caused by the Alpha (B.1.1.7) variant [23].
Another phase 3 trial evaluating the efficacy of NVX-CoV2373 in adults (≥18 years of age) was performed in the United States and Mexico during the first half of 2021 [24]. Two doses of NVX-CoV2373 had an efficacy of 90.4% (95% CI, 82.9–94.6) against COVID-19 and an efficacy of 100% (95% CI, 87.0–100) against moderate-to-severe disease (Table 1).
mRNA vaccines
BNT162b2 and mRNA-1273 are the only two mRNA vaccines listed on the WHO EUL, and are the most widely used COVID-19 vaccines against SARS-CoV-2 infection worldwide [79].
A randomized, double-blind study of the BNT162b2, an mRNA vaccine, performed in a healthy population 16 years of age or older, reported early protection 12 days after the first dose and 95% (95% CI, 90.3–97.6) efficacy 7 days after the second dose (Table 1). Similar vaccine efficacy was observed across subgroups defined by age, sex, race, ethnicity, baseline body-mass index, and the presence of coexisting conditions [25]. During 6 months of follow-up, the vaccine efficacy against COVID-19 was 91.3% (95% CI, 89.0–93.2) and that against severe disease was 96.7% (95% CI, 80.3–99.9) among the participants. In South Africa, where the SARS-CoV-2 variant of concern Beta (B.1.351) was predominant, a vaccine efficacy of 100% (95% CI, 53.5–100) was observed [26]. In a study in participants 12–15 years of age without evidence of previous SARS-CoV-2 infection, no COVID-19 cases with an onset of 7 or more days after dose 2 were observed among BNT162b2 recipients, whereas 16 cases occurred among placebo recipients, thus indicating a vaccine efficacy of 100% (95% CI, 75.3–100).
Injections of two-dose mRNA-1273 28 days apart, as evaluated in a clinical trial at 99 centers across the United States, had a vaccine efficacy in preventing COVID-19 illness of 94.1% (95% CI, 89.3–96.8) for adults ≥18 years of age and 86.4 (61.4–95.2) for people ≥65 years of age [27]. The efficacy in preventing severe disease was 98.2% (95% CI, 92.8–99.6), on the basis of 2 cases in the mRNA-1273 group and 106 in the placebo group; the efficacy in preventing asymptomatic infection starting 14 days after the second injection was 63.0% (95% CI, 56.6–68.5) [28].
The effectiveness of the widely used BNT162b2 vaccine has been reported worldwide, in studies from the United States, United Kingdom, Israel, Italy, Qatar, Canada, France, Hungary, Scotland, South Africa, and others (Table 2). Most of the studies found that the effectiveness of two-dose BNT162b2 vaccination against COVID-19 was high against various variants in adults, including the Delta (B.1.617.2) variant, but decreased over time. However, the two-dose BNT162b2 regimen was found to be less effective against the Omicron (B.1.1.529) variant: for both Omicron (B.1.1.529) BA.1 and BA.2, two doses of BNT162b2 vaccine in the population without previous SARS-CoV-2 infection history showed no protection against symptomatic infections for 200 days post-vaccination. Three doses of the BNT162b2 vaccine conferred 59.6% (95% CI, 52.9–65.3) and 52.2% (95% CI, 48.1–55.9) protection against symptomatic infections caused by the Omicron (B.1.1.529) BA.1 and BA.2 variants within 1 or 2 months, respectively, but greater than 95% protection against severe, critical, or fatal COVID-19 [80]. mRNA-1273 showed high vaccine effectiveness, similar to that of BNT162b2, against symptomatic, severe, critical, or fatal infections caused by various SARS-CoV-2 variants, but mRNA-1273 also showed consistently high protection against the Omicron (B.1.1.529) BA.1 and BA.2 variants (Table 2).
A real-world surveillance study in Slovenia based on data collected from February, 2022 to March, 2022 reported an overall COVID-19 incidence of 98/100,000 in adults 18 years of age or older [81]. The incidence of COVID-19 varied according to vaccination status: 343/100,000 in the unvaccinated population, 132/100,000 in the two-dose mRNA vaccinated population, and 74/100,000 in the three-dose mRNA vaccinated population. In the most vulnerable population, 65 years of age or older, the protection conferred by mRNA vaccines against hospitalization associated with SARS-CoV-2 infection caused by Omicron (B.1.1.529) was 95% (95% CI, 95–96) in three-dose recipients and 82% (95% CI, 79–84) in two-dose recipients. The level of vaccine protection was maintained for at least 6 months.
A case-control study in adolescents aged 12–18 years, performed during the Delta (B.1.617.2) and Omicron (B.1.1.529) epidemics, showed that two-dose BNT162b2 vaccines provided similar protection in the 12- to 15-year-old and 16- to 18-year-old groups (83% versus 82%). The vaccine efficacy during the Delta (B.1.617.2) epidemic was 96% (95% CI, 90–98) and 91% (95% CI, 86–94) among these adolescents for critical cases and non-critical hospitalizations, respectively [82]. The vaccine efficacy during the Omicron (B.1.1.529) epidemic declined to 79% (95% CI, 51–91) for critical cases and to 20% (95% CI, −25–49) for non-critical illness. Although the protection conferred by two doses of BNT162b2 was lower against Omicron (B.1.1.529) than Delta (B.1.617.2) COVID-19 in adolescents, the vaccine protection against critical illness was sustained. In Denmark, a large observational study in adolescents also reported a vaccine effectiveness of 93% (95% CI, 92–94) 60 days post-vaccination with BNT162b2 during the Delta (B.1.617.2) epidemic [83]. Nonetheless, a 3.7-fold (95% CI, 2.7–5.2) lower rate of confirmed infection risk was observed after boosting than after two doses, at time points as long as 60 days post-vaccination in adolescents [84]. All SARS-CoV-2 infections pose a risk of long COVID, but a study has reported that vaccination with mRNA vaccines or Ad26.COV2.S before infection confers only 15% protection against post-acute sequelae, with respect to that in people with SARS-CoV-2 infection without prior vaccination [85].
COVID-19 VACCINES NOT YET ON THE WHO EUL
Some COVID-19 vaccines have not yet been included on the WHO EUL but vaccine efficacy or effectiveness data from trials or observational studies have been reported.
Gam-COVID-Vac (Sputnik V) is a heterologous two-dose regimen against COVID-19 approved for emergency use in Russia, with a recombinant of adenovirus type 26 (rAd26) vector-based vaccine as the first dose, and an rAd5 vector-based vaccine as the second dose; both vectors carry the gene for the full-length SARS-CoV-2 S glycoprotein. Sputnik V provided an overall efficacy of 91.6% (95% CI, 85.6–95.2) and 100% (95% CI, 94.4–100.0) against severe disease in a phase 3 trial in Russia [30]. A vaccination campaign against COVID-19 with the rAd26-rAd5 Sputnik V vaccine, undertaken in Argentina for people older than 60 years, demonstrated an effectiveness of 64% (95% CI, 63–65) against infection, and 93.1% (95% CI, 92.6–93.5) against death during epidemics associated with the Gamma (P.1), Lambda (C.37), and Alpha (B.1.1.7) variants [41] (Table 2). Another retrospective cohort study in Argentina reported an effectiveness of the first component of Sputnik V of 78.6% (95% CI, 74.8–81.7), 87.6% (95% CI, 80.3–92.2) and 84.8% (95% CI, 75.0–90.7) in decreasing SARS-CoV-2 laboratory-confirmed infections, hospitalizations, and mortality, respectively, in an older population 60–79 years of age in a Gamma (P.1) variant dominated period [86].
WIBP-CorV (developed by Wuhan Institute of Sinopharm, China), is a similar β-propionolactone inactivated prototype vaccine to BBIBP-CorV (developed by Beijing Institute of Sinopharm, China) adjuvanted with aluminum hydroxide. The efficacy of WIBP-CorV, as evaluated in the United Arab Emirates and Bahrain among adults 18 years of age or older, demonstrated 72.8% (95% CI, 58.1–82.4) protection against symptomatic COVID-19 at a time when variants were not common [13].
ZF2001, a protein subunit COVID-19 vaccine using the tandem-repeat dimeric RBD of the SARS-CoV-2 spike protein (from the original Wuhan-Hu-1 strain) as an antigen, manufactured by Anhui Zhifei Longcom Biopharmaceutical, has been authorized for emergency use in China. In the recently reported phase 3 trial, a total of 12625 ZF2001 recipients had 158 cases of symptomatic COVID-19, whereas 12568 placebo recipients had 580 cases, thus indicating an efficacy of 75.7% (95% CI, 71.0–79.8) during the Delta (B.1.617.2) predominant period. Three doses of ZF2001 provided 76.0% (95% CI, 71.2–80.1) protection in younger adults 18–59 years of age and 67.6% (95% CI, 21.9–87.8) protection in people over 60 years of age [31]. Three doses of ZF2001 provided 87.6% (70.6–95.7) protection against severe or critical COVID-19.
SCB-2019 is an S protein subunit vaccine against SARS-CoV-2 consisting of the trimeric structure of the S protein adjuvanted with CpG-1018 and alum, developed by Clover Biopharmaceuticals. An efficacy trial was conducted in adults 18 years of age and older in Belgium, Brazil, Colombia, the Philippines, and South Africa from March 24, 2021 to August 10, 2021 [32]. Two doses of SCB-2019 provided 67.2% (95.72% CI, 54.3–76.8) protection against COVID-19 of any severity, 83.7% (97.86% CI, 55.9–95.4) protection against moderate-to-severe COVID-19, and 100% (97.86% CI, 25.3–100.0) against severe COVID-19.
The CoVLP+AS03 vaccine consists of coronavirus-like particles (CoVLP), which are produced in plants and display the prefusion spike glycoprotein of the original strain of SARS-CoV-2, which is combined with an AS03 adjuvant (Adjuvant System 03). In a phase 3 trial, the vaccine efficacy was 69.5% (95% CI, 56.7–78.8) against any symptomatic COVID-19 caused by five variants (Alpha, Gamma, Delta, Lambda, or Mu), 76.9% (95% CI, 51.5–90.0) against moderate disease, and 100.0% (95% CI, -63.7-NA) against severe disease [33].
ZyCoV-D comprises a DNA plasmid vector pVAX1 carrying a gene expressing the spike protein of SARS-CoV-2 and an IgE signal peptide, and is administered intradermally via a needle-free injection system (Table 1). In a multicenter, double-blind, randomized, controlled trial at 49 hospitals in India, ZyCoV-D showed a vaccine efficacy of 66.6% (95% CI, 47.6–80.7) for all COVID-19 cases, 64.9% (95% CI, 44.9–79.8) for mild cases, and 100% for severe cases [34].
CVnCoV is formulated with the RNActive mRNA vaccine platform, containing 12 μg mRNA per dose, which was evaluated in a phase 2b/3 clinical trial in 47 public and private hospitals and clinics across four countries in Europe (Belgium, Germany, the Netherlands, and Spain) and six countries in Latin America (Argentina, Colombia, the Dominican Republic, Mexico, Panama, and Peru) (Table 1). CVnCoV conferred 48.2% (95.826% CI, 31.0–61.4) protection against COVID-19 of any severity and 70.7% (95% CI, 42.5–86.1) protection against moderate-to-severe COVID-19 [35].
BOOSTING IMMUNIZATION WITH COVID-19 VACCINES
Third dose
During the Omicron (B.1.1.529) epidemic, a substantial decrease in vaccine protection occurred. The primary two-dose immunization schedule with BNT162b2, mRNA1273, or ChAdOx1 induced only approximately 50% protection against symptomatic COVID-19 between 14 days and 3 months, whereas the protection against infections was somewhat lower, at approximately 37%. Furthermore, the effectiveness of all three vaccines was below 50% for both symptomatic disease and infections 3 months after the primary series. Similarly, the two-dose regimen of inactivated COVID-19 vaccines also did not protect recipients, regardless of age, against symptomatic COVID-19 [76]. Boosting with a third dose of COVID-19 vaccine, with an mRNA, vector-based vaccine, or inactivated vaccine, provided more than 79% protection against all clinically symptomatic infections within 3 months after booster vaccination [87].
In an observational cohort study in more than 3000 health care workers in the United States, three doses of mRNA vaccines showed an effectiveness of 91% (95% CI, 84–95) in preventing Delta (B.1.617.2) infection, with a relative vaccine effectiveness of 86% (95% CI, 69–94) with respect to the effectiveness of two doses of mRNA vaccine. The effectiveness of two doses of mRNA vaccine was 46% (95% CI, 25–61) in preventing Omicron (B.1.1.529) infection, and the relative vaccine effectiveness was 60% (95% CI, 40–73) [88]. Two- or three-dose vaccination with mRNA vaccines was less effective against mild or asymptomatic infections caused by Omicron (B.1.1.529) than Delta (B.1.617.2). From November 27, 2021 to January 12, 2022, a test-negative design study in England found that booster vaccination with BNT162b2 or mRNA1273 increased vaccine effectiveness against severe disease to more than 75%, and this effect was maintained until approximately 6 months, whereas the effectiveness against symptomatic infections increased to 55–78% within first 3 months after the third dose, then decreased to 29–64% by 3–6 months [46]. Among adolescents 12–15 years of age, a protection of 71.1% (95% CI, 65.5–75.7) was observed 2–6.5 weeks after booster injection of BNT162b2 [89]. Although a BNT162b2 or mRNA-1273 booster dose conferred significantly higher protection against Omicron (B.1.1.529), protection still waned shortly thereafter.
A large-scale prospective cohort study in Chile found that a third dose of the CoronaVac enhanced the vaccine protection against Omicron (B.1.1.529). Compared with only two doses of CoronaVac, the effectiveness of booster vaccination with CoronaVac was 63.8% (95% CI, 60.4–67.0) in preventing laboratory-confirmed SARS-CoV-2 infection, 59.3% (95% CI, 51.5–65.9) in preventing hospitalization, and 62.7% (95% CI, 44.9–74.7) in preventing death [90]. In Tianjin, China, a retrospective study also found that three doses of inactivated vaccines was associated with a significantly lower risk of ICU hospitalization [OR 0.023 (95% CI, 0.002–0.214)], positive nucleic acid re-tests [OR 0.240 (95% CI, 0.098–0.587)], and shorter hospitalization and recovery time [OR 0.233 (95% CI, 0.091–0.596)] in adults with breakthrough infection [91].
Together, this evidence supports the massive boosting vaccination campaign in the previously immunized population to increase protection against COVID-19 diseases associated with Omicron (B.1.1.529) infection.
Fourth dose
Because the protection against Omicron (B.1.1.529) wanes rapidly after the third dose, a fourth dose of COVID-19 vaccine appears necessary to defeat the circulating SARS-CoV-2 variants.
In Israel, one of the first countries to implement national immunization with three doses of COVID-19 vaccines, a study investigating the boosting effects of a fourth dose of COVID-19 vaccine was conducted [92]. The fourth dose, compared with only three doses, decreased the SARS-CoV-2 infection by twofold, and decreased severe disease by 4.3 fold in the older population. Although a fourth dose of BNT162b2 and mRNA-1273 elicited 9–10 fold higher titers of neutralizing antibodies versus baseline before boosting, no significant increases were observed with respect to the peak levels of neutralizing antibodies after the third dose [93]. In addition, a fourth dose had an effectiveness of 30% (95% CI, −8.8–50) with BNT162b2, and 10.8% (95% CI, −43–44) with mRNA-1273 against SARS-CoV-2 infection, with respect to 43.1% (95% CI, 6.6–65.4) with BNT162b2, and 31.4% (95% CI, −18.4–64.2) with mRNA-1273, against symptomatic diseases.
Although a fourth dose of COVID-19 vaccine provides limited additional protection against symptomatic disease associated with SARS-CoV-2 infection, the potential benefits in preventing severe cases or death might be greater in high-risk populations. In Ontario, Canada, a test-negative control database study estimated the relative effectiveness of a fourth dose of vaccination versus three doses among the population 60 years and older living in long-term care centers between December 30, 2021 and March 2, 2022 [94]. The fourth dose of COVID-19 mRNA vaccine enhanced the protection against COVID-19-associated morbidity and mortality caused by the Omicron (B.1.1.529) variant strain in older people in long-term care centers, at an interval more than 84 days after vaccination of the third dose.
Heterologous versus homologous prime-boost vaccination
Various COVID-19 vaccines with different antigens or vaccine vectors have been administered in populations for massive immunization, thus providing a unique opportunity to study heterologous and homologous boost vaccination with the COVID-19 vaccines.
A series of trials or studies have reported that heterologous prime-boost immunization with two different COVID-19 vaccines, particularly a heterologous mRNA vaccine or viral vectored vaccine, as compared with homologous immunization with a single COVID-19 vaccine, elicited greater B cell responses to the pre-fusion sub-structural domains such as RBD, NTD, and greater affinity of the neutralizing antibodies and broader reactivity [95–97].
A nationwide test-negative study in Brazil involving the population of people 18 years of age or older who have received two doses of CoronaVac as a primary immunization followed by a booster dose of CoronaVac or BNT162b2, investigated vaccine protection during the Omicron (B.1.1.529) predominated period [98]. A homologous booster of CoronaVac increased vaccine protection against hospitalization or death, with a relative vaccine effectiveness of 42%, but conferred little or no increase in protection against symptomatic infections. In contrast, a heterologous booster of BNT162b2 significantly increased protection against hospitalization or death, with a relative vaccine effectiveness of 66.9%, which was maintained for at least 3 months. CoronaVac homologous boosting had lower effectiveness against hospitalization or death in people aged 75 years or older (46–54%) than in younger adults, whereas BNT162b2 heterologous boosting elicited high protection in all age groups. These results support heterologous booster vaccination to decrease severe illness and death associated with COVID-19 during the Omicron (B.1.1.529) epidemic.
A test-negative study conducted in the United States between January 2 and March 23, 2022 reported that one dose of Ad26.COV2.S had an effectiveness of 17.8% (95% CI, 4.3–29.5) between 14 days and 1 month, which then decreased to 8.4% (95% CI, 1.5–14.8) at 2–4 months, whereas two-dose Ad26.COV2.S enhanced the protection to 27.9% (95% CI, 18.3–36.5) and 29.2% (95% CI, 23.1–34.8), respectively. One-dose Ad26.COV2.S plus a heterologous booster of mRNA enhanced the vaccine protection to 61.3% (95% CI, 58.4–64.0) and 54.3% (95% CI, 52.2–56.3), respectively, values similar to those induced by three-dose mRNA vaccination [99].
In a population immunized with one dose of adenovirus vaccine, a single dose of an mRNA vaccine heterologous booster provided nearly the same protection as three doses of mRNA vaccine.
A large prospective observational, nationwide cohort study in Chile evaluated the vaccine effectiveness of a booster injection with CoronaVac, AZD1222 or BNT162b2 vaccine in people 16 years of age or older who had completed primary immunization with two doses of CoronaVac [90]. Heterologous boost immunization with BNT162b2 increased the effectiveness to 96.5% (95% CI, 96.2–96.7), and AZD1222 increased the effectiveness to 93.2% (95% CI, 92.9–93.6), as compared with 78.8% (95% CI, 76.8–80.6) after homologous boosting with CoronaVac.
COVID-19 VACCINES IN POPULATIONS WITH UNDERLYING MEDICAL CONDITIONS
Cancer
A retrospective cohort study based on electronic health records of patients with cancer from a multicenter, national database in the United States between December 2020 and November 2021 found a significantly higher risk of breakthrough infection in people with than without cancer after receiving two doses of BNT162b2, or mRNA-1273, or one dose of AZD1222 [100]. Among 45,253 vaccinated patients with 12 specific cancer types, the highest risk was associated with liver cancer [hazard ratio 1.78 (95% CI, 1.38–2.29)], followed by lung cancer [hazard ratio 1.73 (95% CI, 1.50–1.99)], pancreatic cancer [hazard ratio 1.64 (95% CI, 1.24–2.18)], and colorectal cancer [hazard ratio 1.53 (95% CI, 1.32–1.77)]; the lowest risk was for thyroid cancer [hazard ratio 1.07 (95% CI, 0.88–1.30)], skin cancer [hazard ratio 1.17 (95% CI, 0.99–1.38)], breast cancer [hazard ratio 1.16 (95% CI, 1.07–1.25)], and prostate cancer [hazard ratio 1.19 (95% CI, 1.10–1.29)]. Breakthrough infections with SARS-CoV-2 in people with cancer are also associated with a significant and substantial risk of hospitalization and death.
A retrospective, cross-sectional study involving 2,578 patients with cancer from March 2020 to December 2021 was performed to assess the effectiveness of vaccination with BNT162b2 or CoronaVac against COVID-19 [101]. No significant difference in COVID-19 risk between recipients of two doses of BNT162b2 and three doses of CoronaVac vaccine was observed. Two doses of CoronaVac with one boost dose of BNT162b2 was more effective than two doses of BNT162b2 or three doses of CoronaVac; and two doses of BNT162b2 or three doses of CoronaVac provided significantly higher protection than two doses of CoronaVac in these people with cancer.
A population-based test-negative case-control study in 377,194 patients with cancer has indicated that BNT162b2 vaccines had 72.1% effectiveness (95% CI, 71.6–72.7) against COVID-19, whereas the ChAdOx1 nCov-19 vaccine had 59.0% effectiveness (95% CI, 58.5–59.6) [102]. Lower vaccine effectiveness within the prior 12 months was found in patients with cancer, or those who were treated with radiotherapy or systemic anticancer therapy, than in the general healthy population. Furthermore, vaccine effectiveness declined more rapidly in patients with hematologic tumors, such as lymphoma or leukemia, than in patients with solid tumors.
Pregnancy
An observational cohort of 10,861 vaccinated pregnant women 16 years of age or older was matched to 10,861 unvaccinated pregnant controls; the estimated vaccine effectiveness was 96% (95% CI, 89–100) for any documented symptomatic infection, 97% (95% CI, 91–100) for infections with documented symptoms, and 89% (95% CI, 43–100) for COVID-19-associated hospitalization [103]. In addition, vaccination of pregnant people may provide protection against SARS-CoV-2 for newborn children.
Long-term hemodialysis
In 6,076 patients with long-term hemodialysis, the vaccine effectiveness was 68.9% (95% CI, 61.9–74.7) for two-dose BNT162b2 and 66.7% (95% CI, 58.9–73.0) for two-dose mRNA-1273—values lower than those observed in healthy adults [104]. A systematic review reported that 396,062 patients receiving hemodialysis had an elevated risk of COVID-19, with 15-fold greater COVID-19 incidence and associated death risk than that in the general population [105].
Solid organ transplantation
The COVID-19 mRNA vaccines’ effectiveness against COVID-19-associated hospitalizations is diminished in recipients of solid organ transplantation. However, three doses of mRNA vaccine have been found to confer higher protection than two doses of mRNA vaccine among solid organ transplantation recipients. The effectiveness of mRNA vaccines against hospitalization associated with COVID-19 among 440 recipients of solid organ transplantation was 29% (95% CI, -19–58) for a two-dose regimen and 77% (95% CI, 48–90) for a three-dose regimen [106].
In liver transplant patients, the immune response to two doses of BNT162b2 was low, but the third dose significantly improved the humoral and cellular immune response [107]. Further studies are needed to evaluate the persistence of the immune response to three doses in liver transplant patients to determine the optimal number of doses and the interval between the booster dose and the primary doses.
REMAINING KNOWLEDGE GAPS
The ranking of vaccine regimens’ effectiveness has been reported in a living systematic review with network meta-analysis involving a total of 53 studies and 24 combinations of COVID-19 vaccine regimens, with or without boosting, in preventing COVID-19 related symptomatic infection, hospital admission, and death [108]. In this review, a three-dose mRNA COVID-19 vaccine regimen was reported to be the most effective regimen against asymptomatic and symptomatic SARS-CoV-2 infections, with a vaccine effectiveness of 96% (95% CI, 72–99), and against COVID-19 related hospital admission, with an effectiveness of 95% (95% CI, 90–97). Heterologous boosting with two-dose adenovirus vector COVID-19 vaccines plus one booster of mRNA vaccine also showed a satisfactory vaccine effectiveness of 88% (95% CI, 59–97). Lower vaccine effectiveness was noted with two-dose mRNA vaccines, two-dose adenovirus vectored vaccines, one-dose adenovirus vectored vaccines, one-dose mRNA vaccines, and two-dose inactivated vaccines. These data also support that higher protection may be associated with more doses of vaccination and heterologous boosting with COVID-19 vaccines.
However, we should be aware that vaccine effectiveness, particularly the protective effects of COVID-19 vaccines against severe clinical outcomes including severe or critical disease, and death, may be somewhat overestimated because of the “healthy vaccinee bias”: that is, the vaccinated populations in observational studies may potentially be healthier than the unvaccinated population. The vaccinated population’s protection by vaccination, as well as their good health condition, may bias results toward higher vaccine effectiveness. The mRNA vaccine BNT162b2 is used worldwide, with application in immunocompromised populations, and data on vaccine protection in various populations have been collected; however, evidence of the effectiveness of most COVID-19 vaccines in the presence of underlying medical conditions remains limited.
In addition, most reported vaccine efficacy and/or effectiveness findings have been obtained shortly after vaccination. Even with highly effective COVID-19 vaccines, the waning of antibodies over time, as well as the vaccine-induced protection against infection of SARS-CoV-2, may be substantial. In addition, breakthrough infections associated with new emerging variants of SARS-CoV-2 are continually being reported. These emerging strains of the Omicron (B.1.1.529) subtypes, such as BA.4/5, pose a major challenge to the first generation of vaccines based on the antigen of the prototype SARS-CoV-2, as well as the herd immunity against infection with previous SARS-CoV-2 variants [109].
Although receiving a fourth dose of the prototype COVID-19 vaccines may be feasible to restore vaccine protection against SARS-CoV-2, a variant-specific vaccine could theoretically generate more optimal immune memory to both conserved and new epitopes. However, the phenomenon of “original antigenic sin” may inhibit the ability of the vaccine to elicit responses to the new variants [110]. A more ideal solution would be next generation COVID-19 vaccines with a wide epitope coverage to provide cross-immunity against SARS-CoV-2 variants and confer a longer duration of protection.
Efficacy and safety are two core characteristics for vaccines, but in massive administration of vaccines in routine programs, the ease of schedules, vaccine effectiveness, booster need and frequency, cost, factors regarding cold-chain logistics, manufacturing scalability, acceptance by communities, and scope for local or regional production are additional important characteristics. Dr Hanna Nohynek, chair of the COVID-19 Vaccine Working Group of the WHO Strategic Advisory Group of Experts, and Dr Annelies Wilder-Smith, coordinator of the COVID-19 Vaccine Working Group, believe that countries worldwide need multiple vaccines tailored to their national conditions, owing to differences in population structure, clinical practice, and levels of economic development [5]. With more vaccine platforms available, decision-making regarding vaccine selection might be improved, because certain vaccine platforms may be more suitable for specific age groups, subpopulations (e.g., those with underlying immune-compromising or other medical conditions), or pregnant people. Mixing and matching vaccines may increasingly be required to leverage the benefits of each of these platforms.
Although more than 4.6 billion people worldwide have been vaccinated with at least one dose of COVID-19 vaccine approved for use, according to the WHO’s database collected by 200 of 222 countries [111], the remaining knowledge gaps regarding the persistence and spectrum of vaccine protection provided by the currently available COVID-19 vaccines must be investigated in the future. Pancoronavirus COVID-19 vaccines or polyvalent COVID-19 vaccines with broader antigenic composition, improvement of adjuvants, and heterologous prime-boost regimens might provide efficient strategies to confer longer term protection and strengthen the immune response to new SARS-CoV-2 variants.
CONCLUSION
The efficacy or effectiveness of the first generation COVID-19 vaccines observed in clinical trials or real-world studies supports the massive administration of these vaccines in various populations. However, better vaccine protection has been reported in healthy adults than in in older people or those with underlying medical conditions, such as cancer, long-term hemodialysis, or solid organ transplantation. Booster immunization with COVID-19 vaccines is necessary to enhance their effectiveness against SARS-CoV-2, particularly for heterologous prime-boost vaccination.

