Background
Mitral valve (MV) disease is the most common type of valvular disease [1]. Rheumatic and degenerative diseases are common causes of primary MV disease [2]. Morphology assessment of the MV provides an insight into the etiology and severity of valve disease, which is essential for surgical and interventional treatment [3].
Compared with computer tomography (CT), echocardiography can dynamically display the movement of the valve in real-time, and has a higher resolution of the valve. It has become the crucial technique for diagnosing and evaluating the degree of valve disease [4]. Two-dimensional (2D) echocardiography can display the valve through sectional images, which is not conducive to surgeons’ understanding. Three-dimensional (3D) echocardiography technology can reconstruct a 3D virtual valve in just a few minutes, providing a surgical perspective of the MV anatomy. However, both 2D and 3D images on a two-dimensional screen, not a physical model, have limitations in surgical applications [5]. However, 3D printing models, which intuitively display the pathology structure of the annulus and leaflets, could act as a bridge between 2D images and clinical doctors. It can provide the surgeon with a physical model for simulation operation, which is not available in 2D images.
Accordingly, the objectives of our study are twofold: to describe the morphological characteristics of rheumatic and degenerative MV diseases based on 3D printing models and to explore the potential role of 3D printing models on surgical planning and evaluation of MV repair.
Materials and methods
Study population
This study enrolled 30 patients with mitral valvular disease who had pre-procedure transesophageal echocardiography (TEE) at the People’s Hospital of Wuhan University from January 2018 to August 2019 consisting of 12 males and 18 females, aged 32 to 71 years (mean, 57.75 ± 11.10 years). Fifteen cases had rheumatic mitral valve disease (RMVD) and 15 cases had degenerative mitral valve disease (DMVD, including 10 cases of MV prolapse). In addition, a control group of 15 age-matched patients with normal MV anatomy was also studied. Patients with contraindications to TEE and those with any type of cardiac arrhythmia, myocarditis, pericardial, or congenital heart disease were excluded from this study. All patients were in sinus rhythm. The Ethics Committee approved the study protocols at the People’s Hospital of Wuhan University.
3D printing of the silicone MV model
The 3D TEE data were obtained by GE Vivid E9 equipped with a 3- to 8-MHz transesophageal matrix-array transducer. All patients were monitored by synchronized electrocardiogram, and the 3D TEE probe was placed in the middle esophagus to acquire full-volume 3D images. Five consecutive cardiac cycles of the full-volume data set with good quality and clarity were stored and explored to an EchoPac workstation.
The original data in the Digital Imaging and Communications in Medicine format were exported into medical image segmentation software (Materialise Mimics version 19.0; Materialise, Leuven, Belgium). The selected diastolic and systolic images were manually segmented using threshold settings to create a region of interest containing the MV. Subsequently, crop mask was determined by adjusting the sampling frame to include the MV. Trimming and drawing tools were employed to reduce the noise of blood and manually manipulate the shape of the anterior and posterior leaflets. 3D digital models of the MV were then calculated automatically using the calculating 3D function.
Then, the 3D digital models were exported to computer-aided design software (Materialise 3-Matic; Materialise) and refined by the local smoothing tools. Based on the 3D digital models, the boolean subtraction function was utilized to render a mold by offsetting the original MV model by 2 mm. A pipe was added to connect the cavity of the mold for pouring silicone. The 3D mold of the MV models was exported in stereolithography format (.stl) for 3D printing.
The STL file was later transformed to the 3D JG AURORA printer (Shenzhen Aurora Technology Co., Ltd., Shenzhen, China), in which the 3D mold was printed using water-soluble polyvinyl alcohol (ESUN PVA 1.75 mm; ESUN New Materials Co., Ltd., Hubei, China). Then the mold was filled with room temperature vulcanizing (RTV) silicone rubber (Hong Cheng Silicone Products Factory, Guangdong, China) using a syringe. The silicone MV model was removed from the mold once the PVA mold dissolved substantially ( Figure 1 ).
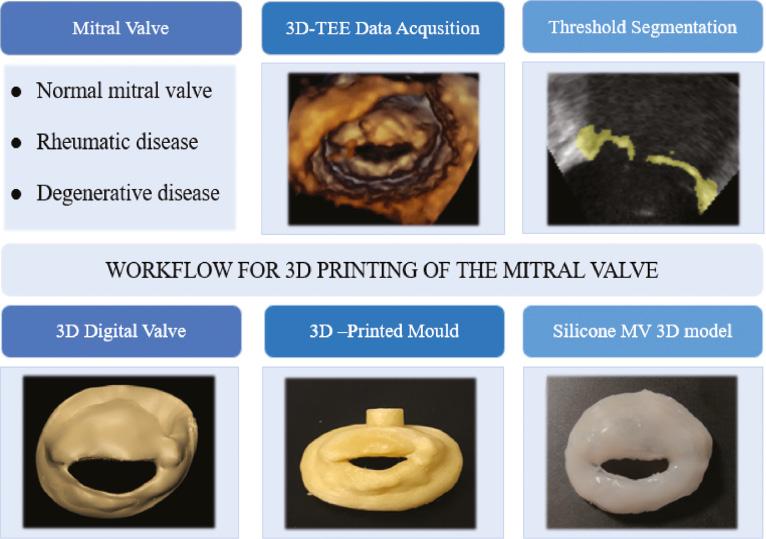
Workflow associated with printing a three-dimensional (3D) silicone model of the mitral valve. A sample of the workflow is shown from the transesophageal acquisition of the 3D echocardiography data set to its segmentation and creation of a virtual 3D model and mold that can be printed using water-soluble polyvinyl alcohol. The 3D model of the valve can be used to appreciate valve morphology and perform qualitative and quantitative analysis useful for pre-procedural planning.
Quantitative assessment of mitral morphology
From 3D printing MV model, several parameters were calculated: annular geometry characteristics, including the anteroposterior (AP) diameter, anterolateral-posteromedial (AL-PM) diameter, commissural width (CW), and annular height (AH), were measured with a vernier caliper. Annular circumference (AC) and area (A) were measured by soft aluminum wire shaping, and the AHCWR was defined as the ratio of annular height to commissural width [6]; leaflet size: the length of the anterior (AL) and posterior (PL) leaflets were measured with a vernier caliper. Soft aluminum wire shaping measured the anterior (AA) and posterior (PA) leaflet areas. The data were analyzed 2 days apart by the same observers (intraobserver) and a different observer (interobserver) blinded to the initial results, and the average value was used for statistical analysis.
Simulated mitral repair on 3D printing model
The MV models of 10 patients with the degenerative disease who intend to undergo MV repairs were set up on a benchtop to simulate operation ( Figure 2 ). Two experienced surgeons were invited to simulate the procedure using our 3D MV model. The mitral repair techniques employed included leaflet resection and ring annuloplasty. Assessments for mitral repair included the coaptation length (CL) and AHCWR in the model valves, and CL was measured as the mean coaptation length of the region where the anterior and posterior leaflets overlap ( Figure 3 ). According to previous studies [7, 8], a strong inverse correlation existed between AHCWR and mitral regurgitation. The mitral regurgitation grades before and after the actual repair were recorded.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 software (2012 release; IBM Corp., Armonk, NY, USA) and Medcalc version 11.0 (Medcalc Software, MedCalc Software Ltd., Ostend, Belgium). Continuous variables were presented as means ± standard deviations, and categorical variables were presented as frequencies or percentages. The intraclass correlation coefficient was used to assess the intraobserver and interobserver variabilities of the 3D printing model morphology measurements. The agreement of CL and AHCWR between the simulated operation and the actual surgery was presented as Bland-Altman plots. Spearman’s correlation analysis and partial correlation analysis were applied to analyze the relationship between mitral regurgitation grades with CL and AHCWR. P < 0.05 was considered statistically significant.
Results
The baseline clinical and echocardiographic characteristics of the patients (15 with RMVD, 15 with DMVD, and 15 controls) are presented in Table 1 . Patients with RMVD and DMVD had a higher New York Heart Association classification than the control group. The left ventricular volumes of the DMVD group were greater that those of the RMVD and control groups, but the ejection fraction of the DMVD group was similar to that of the RMVD and control groups.
Clinical and Echocardiographic Characteristics in Controls and Patients with RMVD and DMVD
| Variable | Controls (n = 15) | RMVD (n = 15) | P | DMVD (n = 15) | P |
|---|---|---|---|---|---|
| Clinical | |||||
| Age (years) | 56.7 ± 11.1 | 58.6 ± 10.9 | 0.231 | 60.7 ± 13.1 | 0.068 |
| Men | 8 (53) | 7 (47) | 0.278 | 9 (60) | 0.198 |
| BSA (m2) | 1.8 ± 0.2 | 1.9 ± 0.5 | 0.089 | 1.8 ± 0.9 | 0.102 |
| NYHA class | <0.001 | <0.001 | |||
| I | 15 (100) | 5 (33) | 4 (27) | ||
| II | 0 (0) | 10 (67) | 11 (73) | ||
| III/IV | 0 (0) | 0 (0) | 0 (0) | ||
| Echocardiographic | |||||
| LVEDV (mL/m2) | 54 ± 8 | 68 ± 10 | <0.001 | 72 ± 8 | <0.001 |
| LVESV (mL/m2) | 21 ± 6 | 25 ± 9 | <0.001 | 28 ± 10 | <0.001 |
| LVEF (%) | 61 ± 10 | 63 ± 14 | 0.372 | 62 ± 16 | 0.110 |
Data are expressed as mean ± standard deviation or number (percentage).
BSA, body surface area; DMVD, degenerative mitral valve disease; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NYHA, New York Heart Association; RMVD, rheumatic mitral valve disease.
The 3D TEE data of all 45 patients were post-processed, and the 3D models were printed out at a 1:1 ratio. Printing time depends on the complexity of the valve anatomy. The 3D reconstruction of digital model was approximately 60 to 85 minutes for each case. Post-processing procedures required 20 to 30 minutes. The mold printing was approximately 3 to 4 hours. Pouring RTV and dissolving mold required 2 to 3 hours.
Morphological analysis on the MV model
Compared with 3D TEE images, the morphological parameters measured on our 3D printing model showed great consistency. No significant differences were found ( Table 2 ). The absolute difference between the 3D printing model and the 3D TEE image indicated that the model’s accuracy was within 2 mm ( Table 3 ). Such results demonstrated that the mold-molding method might be used to produce the patient-specific MV models with high accuracy. The interobserver and intraobserver intraclass correlation coefficients of the measurements of MV morphology were good ( Table 4 ). The geometric parameter of the mitral valve is presented in Table 5 .
Comparison of Measurements Performed on the 3D Printing Model of the MV and on the 3D TEE Image
| Groups | AP (cm) | AL-PM (cm) | AC (cm) | A (cm2) | AL (cm) | PL (cm) |
|---|---|---|---|---|---|---|
| Controls (n = 15) | ||||||
| 3D printing model | 2.89 ± 0.30 | 3.29 ± 0.31 | 10.66 ± 0.82 | 8.05 ± 1.60 | 2.21 ± 0.37 | 1.21 ± 0.30 |
| 3D TEE | 2.97 ± 0.21 | 3.31 ± 0.54 | 10.71 ± 0.43 | 8.01 ± 1.45 | 2.11 ± 0.28 | 1.10 ± 0.24 |
| t | 0.04 | 0.22 | 0.06 | 0.46 | 0.62 | 0.78 |
| P | 0.94 | 0.81 | 0.95 | 0.64 | 0.55 | 0.46 |
| RMVD (n = 15) | ||||||
| 3D printing model | 3.34 ± 0.43 | 3.45 ± 0.60 | 11.10 ± 0.80 | 10.30 ± 1.30 | 2.66 ± 0.28 | 1.44 ± 0.23 |
| 3D TEE | 3.41 ± 0.24 | 3.52 ± 0.64 | 11.21 ± 0.86 | 10.53 ± 1.33 | 2.72 ± 0.38 | 1.33 ± 0.43 |
| t | 0.01 | 0.05 | 0.02 | 0.13 | 0.38 | –0.94 |
| P | 0.96 | 0.95 | 0.98 | 0.88 | 0.72 | 0.37 |
| DMVD (n = 15) | ||||||
| 3D printing model | 3.52 ± 0.71 | 3.98 ± 0.34 | 12.15 ± 1.61 | 11.72 ± 1.10 | 2.38 ± 0.45 | 1.42 ± 0.43 |
| 3D EE | 3.64 ± 0.81 | 3.82 ± 0.42 | 12.23 ± 0.91 | 11.84 ± 0.43 | 2.25 ± 0.55 | 1.31 ± 0.62 |
| t | 0.06 | 0.05 | 0.01 | 0.03 | –0.85 | –0.93 |
| P | 0.94 | 0.95 | 0.99 | 0.97 | 0.42 | 0.38 |
3D, three-dimensional; AP, antero-posterior diameters; AL-PM, anterolateral and posteromedial diameters; AC, annular circumferences; A, annular area; AL, length of anterior leaflet; PL, length of posterior leaflet; TEE, transesophageal echocardiography.
Comparison of Absolute Difference between the 3D Printing Model and 3D the Transesophageal Echocardiography Parameters
| Groups | AP (cm) | AL-PM (cm) | AC (cm) | A (cm2) | AL (cm) | PL (cm) |
|---|---|---|---|---|---|---|
| Controls | 0.09 ± 0.07 | 0.06 ± 0.02 | 0.07 ± 0.04 | 0.08 ± 0.05 | 0.07 ± 0.05 | 0.09 ± 0.05 |
| RMVD | 0.05 ± 0.01 | 0.09 ± 0.06 | 0.12 ± 0.06 | 0.13 ± 0.06 | 0.05 ± 0.04 | 0.13 ± 0.04 |
| DMVD | 0.12 ± 0.03 | 0.16 ± 0.04 | 0.10 ± 0.05 | 0.11 ± 0.05 | 0.15 ± 0.03 | 0.12 ± 0.08 |
3D, three-dimensional; A, annular area; AC, annular circumferences; AL, length of anterior leaflet; AL-PM, anterolateral and posteromedial diameters; AP, anteroposterior diameters; DMVD, degenerative mitral valve disease; PL, length of posterior leaflet; RMVD, rheumatic mitral valve disease; TEE, transesophageal echocardiography.
Strength of Agreement of the Interobserver and Intraobserver Analyses for Each Mitral Valve Parameter Measured on Three-dimensional Printing Model
| Parameters | Intraclass Correlation Coefficients | |
|---|---|---|
| Intraobserver | Interobserver | |
| AP | 0.842 | 0.812 |
| AL-PM | 0.902 | 0.897 |
| CW | 0.817 | 0.810 |
| AC | 0.942 | 0.932 |
| A | 0.923 | 0.897 |
| AH | 0.934 | 0.923 |
| AHCWR | 0.916 | 0.903 |
| AL | 0.926 | 0.917 |
| PL | 0.893 | 0.867 |
| AA | 0.876 | 0.865 |
| PA | 0.925 | 0.915 |
A, annular area; AA, anterior leaflet area; AC, annular circumference; AH, annular height; AHCWR, annular height to commissural width ratio; AL, length of anterior leaflet; AL-PM, anterolateral and posteromedial diameters; AP, anteroposterior diameters; CW, commissural width; PA, area of posterior leaflet; PL, length of posterior leaflet.
Measurements Obtained from the Three-dimensional Printing Model of the MV in Controls and in Patients with RMVD and DMVD
| Controls (n = 15) | RMVD (n = 15) | DMVD (n = 15) | |
|---|---|---|---|
| Annulus | |||
| AP (cm) | 2.89 ± 0.30 | 3.34 ± 0.43a | 3.52 ± 0.71a |
| AL-PM (cm) | 3.29 ± 0.31 | 3.45 ± 0.60 | 3.98 ± 0.34a |
| CW (cm) | 3.23 ± 0.41 | 3.42 ± 0.54 | 3.88 ± 0.21a |
| AC (cm) | 10.66 ± 0.82 | 11.10 ± 0.80 | 12.15 ± 1.61ab |
| A (cm2) | 8.05 ± 1.60 | 10.30 ± 1.30 | 11.72 ± 3.10a |
| AH (cm) | 0.73 ± 0.19 | 0.70 ± 0.15 | 0.58 ± 0.17a |
| AHCWR (%) | 23.00 ± 6.00 | 21.00 ± 5.00 | 15.00 ± 4.00ab |
| Leaflets (mm2) | |||
| AL (cm) | 2.21 ± 0.37 | 2.66 ± 0.28a | 2.38 ± 0.45ab |
| PL (cm) | 1.21 ± 0.30 | 1.44 ± 0.23 | 1.42 ± 0.52a |
| AA (cm2) | 5.44 ± 1.89 | 6.77 ± 1.19a | 6.27 ± 1.74 a |
| PA (cm2) | 4.05 ± 0.95 | 4.94 ± 2.03 | 5.69 ± 2.71 a |
Data are expressed as mean ± standard deviation. a P < 0.05 versus control subjects. b P < 0.05 versus RMVD.
A, annular area; AA, anterior leaflet area; AC, annular circumference; AH, annular height; AHCWR, annular height to commissural width ratio; AL, length of anterior leaflet; AL-PM, anterolateral and posteromedial diameters; AP, anteroposterior diameters; CW, commissural width; DMVD, degenerative mitral valve disease; PA, area of posterior leaflet; PL, length of posterior leaflet; RMVD, rheumatic mitral valve disease.
Compared with the control group, patients of the RMVD group had larger AL and AA (P < 0.05); the AP, ALPM, CW, AH, AHCWR, AC, A, PL, and PA were not significantly different from those of the normal group (P > 0.05). These measurements demonstrated that the annulus was not significantly expanded or flat, and the anterior leaflet became lengthier and larger. The 3D printing model can reflect the thickened and fused leaflets and granular calcification ( Figure 4 ).
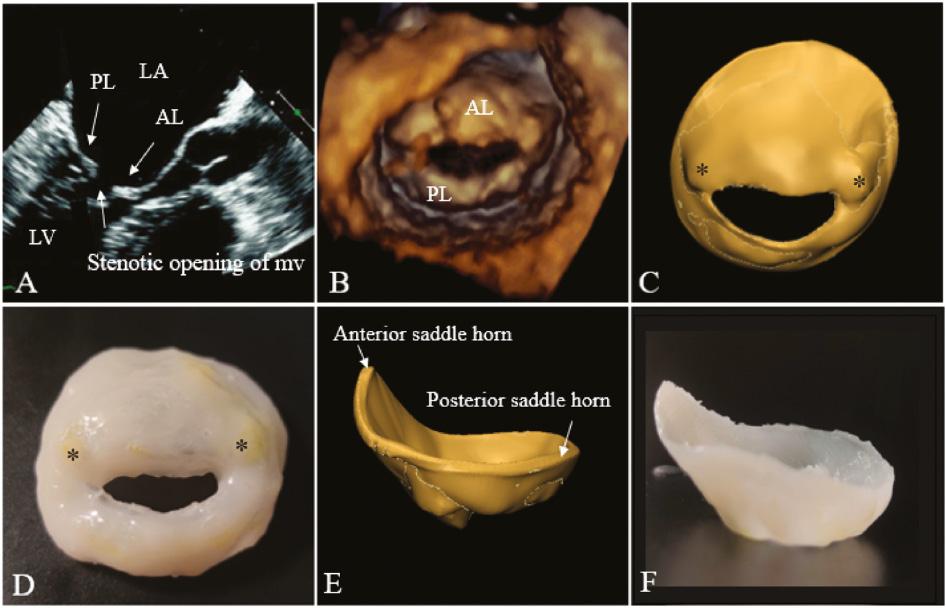
2D and 3D TEE images and virtual and silicone valve models of a patient with RMVD. (A) Long-axis view of the left ventricle and atrium during diastole. The relative positions of the AL and PL are indicated. (B) 3D TEE model. Virtual (C) and silicone (D) models viewed from the left ventricle, the stenotic opening of mitral valve, thickened valves, and yellow granular calcification (*) can be reflected. Virtual (E) and silicone (F) models as viewed in profile from the anterior to the posterior commissures. 2D, two-dimensional; 3D, three-dimensional; AL, anterior mitral valve leaflet; PL, posterior mitral valve leaflet; RMVD, rheumatic mitral valve disease; TEE, transesophageal echocardiography.
Patients of the DMVD group had dilated mitral annulus with significantly increased AP, ALPM, CW, AC, and A (P < 0.05), and the reduction in AH and AHCWR (P < 0.05) represented the flattening of the mitral annulus. The length and area of anterior and posterior leaflets increased significantly in the DMVD groups (P < 0.05). The 3D printing model demonstrated that the annulus diameter was significantly larger than that of the RMVD group, and it showed the location and extent of the prolapsed leaflet ( Figure 5 ).
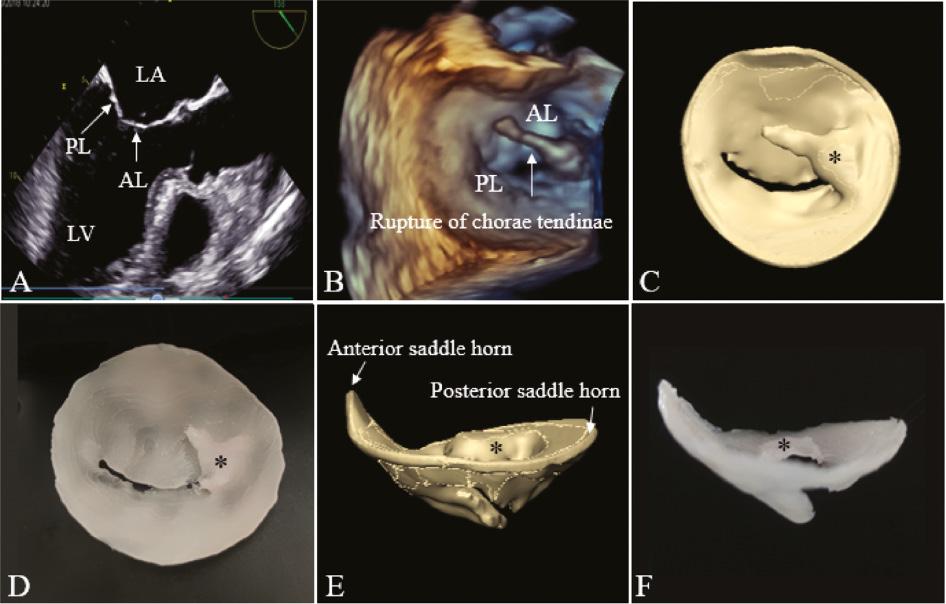
2D and 3D on TEE images and virtual and silicone valve models of a patient with P3 leaflet prolapse. (A) Long-axis view of the left ventricle and atrium during systole. The relative positions of the AL and PL are indicated. (B) 3D TEE model. Virtual (C) and silicone (D) models viewed from the left atrium; P3 leaflet prolapse (*) and rupture of chordae tendineae can be seen. Virtual (E) and silicone (F) models as viewed in profile from the anterior to the posterior commissures, in which the flat annulus can be appreciated (versus Figure 4E, F ). 2D, two-dimensional; 3D, three-dimensional; AL, anterior mitral valve leaflet; PL, posterior mitral valve leaflet; TEE, transesophageal echocardiography.
Mitral repair simulations on the 3D printing model
Two experienced surgeons performed MV repair on 10 models ( Figure 6 ; Table 6 ). The increases in CL and AHCWR in the models were similar those of the actual patient mitral repair ( Table 7 ). Bland-Altman plots demonstrated good agreement between these two parameters in the simulated operations on the 3D printing model and the actual surgery on the patient valve ( Figure 7 ). The strongest correlation with the degree of MR was observed with CL (r = –0.87, P < 0.01; Figure 8A ). The higher degree of MR was also related to lower AHCWR (r = –0.79, P < 0.01; Figure 8B ).
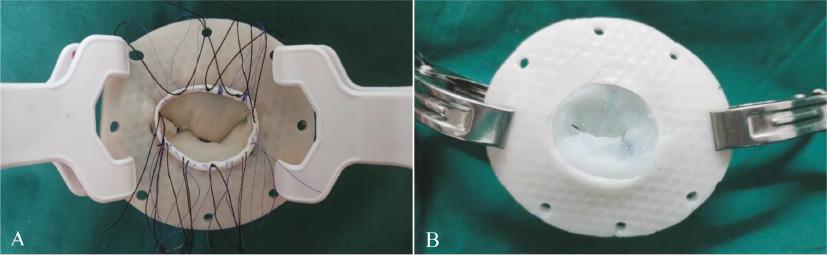
Variety of repairs performed on models: (A) annuloplasty ring being parachuted down onto the model and (B) suturing the posterior leaflet of a model during a triangular tissue resection repair.
Mitral Repair Completed on Model and Patient Valves
| Surgical Plan | Model Valve (n = 10) | Patient Valve (n = 10) |
|---|---|---|
| Triangular resection | 3 (30) | 4 (40) |
| Rectangle resection | 3 (30) | 2 (20) |
| Commissure closure | 1 (10) | 2 (20) |
| Edge-to-edge repair | 1 (10) | 1 (10) |
| Folding repair | 2 (20) | 2 (20) |
| Annuloplasty ring | 10 (100) | 10 (100) |
Data are expressed as number (percentage).
Measurements of the Patient and Model Valves Before and After Mitral Valve Repair
| Patient | Model Valve | Patient Valve | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coaptation Length (cm) | AHCWR (%) | Coaptation Length (cm) | AHCWR (%) | Regurgitation Grade | ||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| A | 0.49 | 0.84 | 19.60 | 23.40 | 0.53 | 0.88 | 19.20 | 23.60 | 3 | 1 |
| B | 0.51 | 0.89 | 15.60 | 21.20 | 0.55 | 0.94 | 16.30 | 21.60 | 3 | 1 |
| C | 0.45 | 0.96 | 14.90 | 20.50 | 0.44 | 0.87 | 14.30 | 20.90 | 4 | 1 |
| D | 0.45 | 0.87 | 15.50 | 20.50 | 0.43 | 0.90 | 15.30 | 20.80 | 4 | 1 |
| E | 0.40 | 0.78 | 11.50 | 20.90 | 0.47 | 0.80 | 11.30 | 20.50 | 4 | 2 |
| F | 0.47 | 0.95 | 18.40 | 24.50 | 0.50 | 0.91 | 19.80 | 24.80 | 3 | 1 |
| G | 0.48 | 0.83 | 16.20 | 22.40 | 0.51 | 0.85 | 17.30 | 22.90 | 3 | 1 |
| H | 0.39 | 0.76 | 12.40 | 20.40 | 0.42 | 0.78 | 12.90 | 20.30 | 4 | 2 |
| I | 0.42 | 0.85 | 13.60 | 20.10 | 0.46 | 0.86 | 14.80 | 20.40 | 4 | 2 |
| J | 0.43 | 0.88 | 14.30 | 20.30 | 0.48 | 0.89 | 15.30 | 20.90 | 4 | 1 |
| Mean ± standard deviation | 0.45 ± 0.04 | 0.86 ± 0.06 | 15.20 ± 2.49 | 21.42 ± 1.51 | 0.48 ± 0.04 | 0.87 ± 0.05 | 15.65 ± 2.63 | 21.67 ± 1.56 | 3.60 ± 0.52 | 1.30 ± 0.48 |
| P | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |||||
AHCWR, annular height to commissural width ratio; pre, pre-repair; post, post-repair.
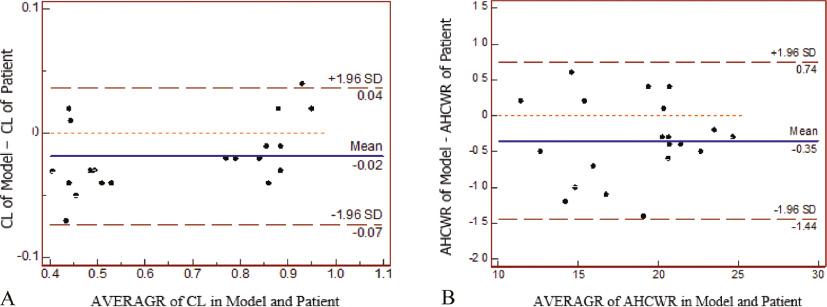
Bland-Altman plots showing agreement in the measurements obtained in the simulated operations on three-dimensional printing model and the actual surgery on patient valve. (A) Length of the coaptation. (B) Ratio of the annular height to the commissural width (AHCWR).

Correlations of regurgitation grade with coaptation length and annular height to commissural width ratio (AHCWR). The scatterplot demonstrates an inverse correlation between regurgitation grade and coaptation length (A) and an inverse correlation between regurgitation grade and AHCWR (B).
The model of patient C underwent two different repair strategies to evaluate the effects of the two strategies and choose superior surgical planning. Patient C had posterior leaflet and commissural prolapse and underwent repair by prolapse leaflet rectangle resection and ring annuloplasty and by posterior leaflet triangular resection, commissure closure, and ring annuloplasty. Both mitral repair strategies resulted in increasing leaflet coaptation; however, the latter had a superior effect on increasing the coaptation length and restoring the shape of the annulus as opposed to the former (CL, 0.85 versus 0.96 cm; AHCWR, 18.9% versus 20.5%).
Discussion
In our experiments, the principal results are as follows: the 3D printing model could intuitively display the morphological characteristics of the rheumatic and degenerative mitral disease and provide quantitative anatomical information for the clinic; (2) the silicone 3D printing model could be applied to mitral repair simulation, providing decision-making information for the clinic based on morphological changes after surgery.
We observed morphological characteristics of rheumatic and degenerative mitral disease based on the 3D printing model. Both the RMVD and DMVD groups showed significant differences in the MV structure compared with the normal group. In the RMVD group, the difference mainly lay in the leaflets, with the AL and AA increasing. Adhesion at the junction, calcification, and thickening on leaflets as well as chordae fusion shortening restricted the leaflets’ opening and the annulus’ movement or expansion [9]. Meanwhile, in the DMVD group, the AP, ALPM, CW, AC, and A increased, and parameters of AH and AHCWR reduced obviously, indicating that the original “saddle” shape of the MV annulus became flat and dilated. Previous studies have found that AHCWR can reflect the 3D degree of the annulus, and a smaller value indicates that the annulus becomes flat [10], and the group of DMVD had increased leaflet area of anterior as well as posterior.
Although many methods for quantitative assessment of MV anatomy exist [11], the 3D printing model of the MV not only could quantitatively evaluate the morphological characteristics of the annulus and leaflets but could also intuitively display the pathology structure of the annulus and leaflets, which were the most important basis for choosing between MV repair and replacement [12]. Mitral repair remains the standard treatment for severe mitral regurgitation with better survival rates, better preservation of the left ventricle function, and lower complications [13, 14]. However, mitral repair involves many procedures, which are becoming more refined, individualized, and localized, and the MV’s reparability depends on patient anatomy and surgeon experience [15]. Therefore, the more important advantage of the 3D printing model was that it could provide preoperative simulation, assist in the surgical decision-making, and improve the success rate of surgery, which cannot be provided by quantitative software evaluation.
To simulate mitral repair before the actual operation, our research used silicone as material, which was most suitable for simulating the material properties of the valve [16], but the silicone could not be used in the 3D printer directly. Therefore, the silicone valve models were generated by creating molds printed with water-soluble polyvinyl alcohol, then filled with RTV silicone. This method was widely used in 3D printing of valve and blood vessels and proved the superior material properties of silicone valve for simulation purposes [17].
In our research, we performed the mitral repair operation simulation on the model of 10 patients. The primary goal of mitral repair surgery was to increase leaflet coaptation and prevent further dilatation of the annulus [8]. A previous study has shown [7] that coaptation length and AHCWR were related to the degree of mitral regurgitation. Thus, our study evaluated the effect of mitral repair by comparing the measurement of the CL and AHCWR before and after repair. The simulated operation on the model was consistent with the measured value in the actual operation, indicating that the ability of the models to simulate mitral repair conditions and results similar to those experienced clinically by the patients. The correlation between the degree of MR with CL (r = –0.87, P < 0.01) and AHCWR (r = –0.79, P < 0.01) had also been confirmed in our research. More importantly, we could simulate multiple repair plans on the 3D printing model for some patients with complicated repairs and select the superiority of one strategy by morphological evaluation.
Limitations
There were several limitations in our study. The 3D printing model in our study mainly contained the structure of leaflets and annulus, whereas the chordae and papillary muscles were parallel to the ultrasound beam during imaging and could not be reconstructed well [18]. Therefore, the assessment and repair of the chordae and papillary muscle in MV disease was. Besides, the heart valves were a dynamic structure; the MV model we created was a static model and thus cannot reflect the hemodynamic changes of the valve [19]. A study [20] assessed the 3D printing model dynamically combined with a phantom heart simulator. Moreover, the 3D printing model could reflect the morphological characteristics of RMVD, but the repair of RMVD was complicated. The patients in this study finally underwent MV replacement. Therefore, the application prospect of 3D printing models in RMVD requires us to explore further.
Conclusion
3D printing models of the MV from 3D TEE data could be used in the morphological analysis of the MV before and after surgery in DMVD, and these models could potentially assist in personalized mitral repair, in the evaluation the effectiveness of mitral repair based on morphological changes, and in improving the efficiency of complex mitral repairs and the ability of inexperienced surgeons.



