Introduction
According to the 2006 European League Against Rheumatism guidelines, hyperuricemia is defined as an elevated serum urate level exceeding 6.0 mg/dL [1, 2]. Hyperuricemia can lead to monosodium urate formation and accumulation in joints or other tissues, and can cause gout [3]. Hyperuricemia is a risk factor for hypertension, insulin resistance, kidney disease, and cerebro-cardiovascular disease [4–6].
Commonly used urate-lowering drugs in China, such as allopurinol, benzbromarone, or febuxostat, are associated with differing adverse effects. Allopurinol can cause severe cutaneous adverse reactions and hypersensitivity, because of the predisposing genetic factor HLA-B*5801, particularly in Asian populations [7–9]. Benzbromarone has been withdrawn from many European markets because of hepatotoxicity and deaths due to hepatic failure [7]. Febuxostat, compared with allopurinol, is associated with a greater risk of cardiovascular death in patients with gout [10]. Topiroxostat (FYX-051, CAS# 577778-58-6, Fuji Yakuhin Co., Ltd), a xanthine oxidoreductase inhibitor, has demonstrated good safety and is approved to treat hyperuricemia and gout in Japan [11–13]. Topiroxostat 120 mg/day has shown non-inferior efficacy to that of allopurinol at 200 mg/day [11]. Additionally, Topiroxostat has demonstrated other effects, such as renoprotective effects [14, 15]. Given the good safety and efficacy of Topiroxostat, this drug should be introduced in China.
Previous clinical trials in the Japanese population have revealed that Topiroxostat shows a regular pharmacokinetic (PK) profile. However, differences in genetic background between the Japanese and Chinese populations might potentially influence the PK or drug response [16, 17]. To our knowledge, no reports have described the PK and pharmacodynamic (PD) characteristics of Topiroxostat in Chinese participants. Therefore, to evaluate the PK profile, safety, and efficacy in the Chinese population, we designed and performed this phase I, single-ascending-dose, multiple-dose, and food-effect trial of Topiroxostat in healthy Chinese participants.
Methods
Participants
A total of 30 healthy Chinese adults were enrolled in this study from October 28th to November 17th in 2020. The inclusion criteria were as follows: ≥18 and ≤45 years of age; weight: ≥50.0 kg for men or ≥45.0 kg for women; body mass index (BMI) ≥19.0 and ≤25 kg/m2; no abnormalities in vital signs, physical examination, hematology analysis, urinalysis, chemistry, coagulation test, electrocardiogram, and liver color Doppler ultrasound; voluntarily participation in the study, provision of signed informed consent, and willingness and ability to comply with trial procedures; and no pregnancy plans, and voluntary use of effective contraceptive measures during treatment. The major exclusion criteria were as follows: asthma, urticaria, and other allergic disease history, or allergy to Topiroxostat or related pharmaceutic adjuvants; presence of acute illness within 2 weeks prior; history of serious diseases affecting important organs or systems (including but not limited to the liver, kidneys, heart, lungs, immune, gastrointestinal tract, skin, or metabolic system); history of gout; history of taking any medicine, herbs, or other drugs within 2 weeks prior; history of taking mercaptopurine-associated drugs within 4 weeks prior; history of consumption of alcohol, caffeine, grapefruit, or other foods that might influence drug metabolism in 48 h prior; positive blood pregnancy test; and poor compliance.
This trial was approved by the ethics committee of Sun Yat-sen University Memorial Hospital (2020-YW-061). Additionally, this trial was registered on www.chinadrugtrials.org.cn (CTR20202265). Written informed consent was obtained from all participants.
Study Endpoint, Safety, and Efficacy Assessments
The major endpoints of this trial were the PK profiles obtained in single-ascending-dose, multiple-dose, and food-effects studies in healthy Chinese adults, including Cmax, AUC0-t, AUC0-∞, Tmax, t1/2, MRT0-t, MRT0-∞, CL/F, Vz/F, and Kel.
Minor endpoints were the assessment of the safety and efficacy of Topiroxostat. Efficacy was evaluated on the basis of plasma uric acid concentration (ΔECmax, change in maximum uric acid effective concentration; ΔAUEC: change in area under the uric acid concentration-time curve), determined by the Laboratory Department, Sun Yat-sen Memorial Hospital. Assessments of adverse events (AEs) and safety assessments were performed by clinicians.
Trial design
To evaluate the safety, PD, PK profile, and food effects of Topiroxostat (Fuji Yakuhin Co., Ltd.), we designed and performed this phase I, single-site, randomized, single-ascending-dose, multiple-dose, and food-effect trial ( Figure 1 ) by using 20 mg tablets of Topiroxostat.
This trial consisted of three arms ( Figure 1 ), as follows.
1st arm: single-dose arm. A single-ascending-dose, random, open-label, parallel trial was designed to evaluate a single dose of Topiroxostat. Doses of 20 mg, 40 mg, and 80 mg were administered to ten participants (fasting; male:female ratio = 1:1). Because the protocol for the 40 mg group in the single-dose arm was the same as that for the 40 mg fasting subgroup, data for the 40 mg group in the single-dose arm were collected from the food-effects arm ( Figure 1 ).
The 40 mg group PK data were collected in the food-effects arm.
A total of 17 time points of blood for PK analysis were sampled pre-administration, and at 0.083 h (5 min), 0.25 h (15 min), 0.50 h (30 min), 0.75 h (45 min), 1.00 h, 1.25 h, 1.50 h, 2.00 h, 2.50 h, 3.00 h, 4.00 h, 6.00 h, 8.00 h, 12.00 h, 24.00 h, and 48.00 h.
Three time points of blood for PD analysis were sampled pre-administration, and at 12 h and 24 h.
2nd arm: food-effects arm. A single dose, random, open label, double-cycle, cross-over trial was designed to evaluate food effects (standardized high-fat meal) on Topiroxostat (40 mg). The high-fat diet contained a total of 926.6 kcal with 50% fat. The high-fat menu included milk (250 mL), egg (approximately 110 g), toast (100 g), bacon (55 g), and butter (21 g). We enrolled ten participants, who were randomly divided into two groups. The 40 mg fasting group data were the same as those in the 1st arm ( Figure 1 ). The wash-out time between the fed and fasting intervention was more than 4 days.
A total of 17 time points of blood for PK analysis per cycle was sampled pre-administration, and at 0.083 h (5 min), 0.25 h (15 min), 0.50 h (30 min), 0.75 h (45 min), 1.00 h, 1.25 h, 1.50 h, 2.00 h, 2.50 h, 3.00 h, 4.00 h, 6.00 h, 8.00 h, 12.00 h, 24.00 h, and 48.00 h.
Three time points of blood for PD analysis were sampled per cycle pre-administration, and at 12 h and 24 h.
3rd arm: multiple-dose arm. After more than 4 days of wash-out, the ten participants who received 80 mg Topiroxostat in the 1st arm were enrolled into the multiple-dose (3rd) arm. They received 80 mg Topiroxostat twice per day (q12 h) from the morning of day 1 to day 7 (a total of 13 times).
A total of 36 time points of blood for PK analysis were sampled pre-administration, and at 0.083 h (5 min), 0.25 h (15 min), 0.50 h (30 min), 0.75 h (45 min), 1.00 h, 1.25 h, 1.50 h, 2.00 h, 2.50 h, 3.00 h, 4.00 h, 6.00 h, 8.00 h, 12.00 h, 48 h, 72 h, 96 h, 120 h, 144 h, 144.083 h, 144.25 h, 144.50 h, 144.75 h, 145.00 h, 145.25 h, 145.50 h, 146.00 h, 146.50 h, 147.00 h, 148.00 h, 150.00 h, 152.00 h, 156.00 h, 168.00 h, and 192.00 h.
Topiroxostat tablets were taken orally by all participants with 240 mL of warm water (40–45°C).
Quantification of Topiroxostat
Blood samples (4 mL per time point) were collected into heparin sodium polypropylene tubes. Plasma was separated by centrifugation at 2–8°C and 1,500 g for 10 min. All samples were frozen at −80°C in 2 h. The analytes were extracted by protein precipitation.
The plasma concentration of Topiroxostat was detected by liquid chromatography and tandem mass spectrometry with D4-Topiroxostat (CATO Research Chemicals In.) as the internal standard. The range of quantification was 1.00–1000 ng/mL. A SHIMADZU LC-20A system equipped with a ZORBAX Extend C18 (4.6×150 mm, 3.5 μm, Agilent) column and Security Guard Cartridges C18 (4×2.0 mm ID, Phenomenex) pre-column were maintained at 40°C. The mobile phase was 5 mM ammonium acetate and acetonitrile (v:v = 3:2) at 1.0 mL/min for 3.0 min. An AB SCIEX Turbo Spray/4000 Q TRAP system was used to detect the analyte. The ESI negative mode ion spray voltage was set to −4.2 kV at 500°C.
The linear range of this method was 1.00–1000 ng/mL. The accuracy of intra-day precision (including LLOQ) ranged from 96.5% to 105.1%. Additionally, the accuracy of the inter-day precision (including LLOQ) was in the range of 98.9%–101.9%. The matrix effects, recovery, and stability results are shown in Supplement Tables 2–5. No clear hyperlipidemia matrix effect was observed in the low-concentration quality control (deviation: −3%) and the high-concentration quality control (deviation: 0.88%).
Statistical analysis
The PK parameter set was used for analysis of all PK data. PK and PD parameters were calculated in Phoenix WinNonlin 8.1. An analysis of variance model was used to determine the effect of food on PK. The safety set (SS) was used for AE analysis. The PD parameter set was used for the analysis of PD data. Quantitative data are described as mean ± SD and number. Qualitative data are described as frequency and constituent ratio.
Other statistical analyses were performed in SPSS version 21.0 (IBM®). Categorical variables were analyzed with χ2 test or Fisher exact test. Continuous variables were analyzed with Mann-Whitney U-test to compare two subgroups. P values < 0.05 were considered to be statistically significant.
Results
Characteristics of participants
A total of 30 healthy Chinese adults were enrolled in this study. All participants were included in the PKPS, PD parameter set, and SS analysis. The baseline characteristics of the 30 participants are summarized in Table 1 . No significant differences were observed in the characteristics of participants among the three arms.
Characteristics of Participants
| Characteristics | 20 mg (N = 10) | 40 mg (N = 10) | 80 mg (N = 10) | Total (N = 30) | ||
|---|---|---|---|---|---|---|
| Fasting | Fed | Total | ||||
| Age (years, mean ± SD) | 24.95 ± 4.29 | 26.97 ± 5.02 | 28.86 ± 3.81 | 27.91 ± 4.32 | 27.12 ± 5.28 | 26.66 ± 4.67 |
| Sex: Male/female | 5/5 | 3/2 | 2/3 | 5/5 | 5/5 | 15/15 |
| Ethnicity: Han/Other | 10/0 | 5/0 | 4/1 | 9/1 | 9/1 | 28/2 |
| Height (cm, mean ± SD) | 162.85 ± 7.34 | 169.60 ± 10.37 | 168.30 ± 12.99 | 168.95 ± 11.10 | 161.70 ± 10.11 | 164.50(9.86) |
| Weight (kg, mean ± SD) | 55.04 ± 6.52 | 64.18 ± 11.33 | 62.06 ± 13.37 | 63.12 ± 11.73 | 58.73 ± 7.32 | 58.96(9.16) |
| BMI (kg/m2, mean ± SD) | 20.69 ± 1.25 | 22.14 ± 1.63 | 21.72 ± 2.28 | 21.93 ± 1.88 | 22.42 ± 1.22 | 21.68(1.61) |
PK and PD characteristics in the single-ascending-dose arm
PK parameters are summarized in Table 2 . The drug-time curve of the single-ascending-dose arm is illustrated in Figure 2A . Cmax, AUC0-t, and AUC0-∞ increased with ascending dose. The linear analysis of dose and Cmax, AUC0-t, and AUC0-∞ is shown in Figure 2B (R2 = 0.5146, 0.8416, and 0.8386, respectively). AUC0-t and the AUC0-∞ showed a linear relationship with dose.
PK Parameter Summary
| PK Parameter | 20 mg | 40 mg | P* | 80 mg | P # | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | Fasting | Fed | Fasting | First Dose | Last Dose | |||||||||
| Mean | CV(%) | Mean | CV(%) | Mean | CV(%) | Mean | CV(%) | Mean | CV(%) | Mean | CV(%) | |||
| Cmax, Css_max (ng/mL) | 331.08 | 55.2 | 478.40 | 36.7 | 316.00 | 43.0 | 0.033 | 1285.90 | 59.1 | 673.70 | 30.5 | 1032.40 | 47.3 | 0.046 |
| Css_min (ng/mL) | / | / | / | / | / | / | / | / | / | / | / | 6.22 | 54.0 | / |
| Css_av (ng/mL) | / | / | / | / | / | / | / | / | / | / | / | 122.51 | 29.3 | / |
| AUC0-t (h·ng/mL) | 333.35 | 28.9 | 629.60 | 20.8 | 530.49 | 25.4 | 0.112 | 1734.96 | 33.4 | 1278.50 | 26.0 | 1563.83 | 29.5 | 0.130 |
| AUC0-∞ (h·ng/mL) | 343.11 | 27.7 | 643.77 | 22.2 | 538.91 | 25.4 | 0.111 | 1753.77 | 33.2 | 1304.29 | 26.6 | 1620.46 | 32.1 | 0.127 |
| Tmax (h) | 0.65 | 94.6 | 1.18 | 62.7 | 1.58 | 48.5 | 0.249 | 0.80 | 60.4 | 1.60 | 30.6 | 1.45 | 34.5 | 0.507 |
| t1/2 (h) | 3.72 | 109.5 | 6.08 | 111.5 | 2.49 | 139.4 | 0.153 | 3.31 | 31.2 | 1.73 | 49.0 | 5.82 | 75.8 | 0.010 |
| MRT0-t (h) | 1.88 | 46.7 | 2.51 | 33.5 | 2.40 | 24.3 | 0.737 | 2.44 | 25.0 | 2.33 | 21.2 | 3.49 | 23.2 | 0.001 |
| MRT0-∞ (h) | 2.55 | 65.0 | 3.23 | 37.0 | 2.67 | 29.0 | 0.230 | 2.73 | 23.9 | 2.56 | 22.3 | / | / | |
| CL/F (L/h) | 62.34 | 27.3 | 64.85 | 21.7 | 77.72 | 20.0 | 0.069 | 51.54 | 43.2 | 66.88 | 37.6 | 59.75 | 38.3 | 0.516 |
| Vz/F (L) | 336.36 | 119.3 | 491.48 | 91.9 | 250.21 | 113.8 | 0.170 | 242.80 | 44.8 | 154.67 | 35.2 | 450.81 | 53.2 | 0.001 |
| Kel (1/h) | 0.36 | 72.6 | 0.22 | 62.3 | 0.49 | 45.6 | 0.004 | 0.22 | 21.6 | 0.46 | 32.3 | 0.17 | 64.7 | <0.001 |
| AUC_%Extrap (%) | 3.07 | 75.7 | 1.96 | 92.0 | 1.55 | 66.6 | 0.541 | 1.10 | 44.1 | 1.81 | 80.6 | 3.04 | 77.3 | 0.177 |
PK, pharmacokinetic; *fasting group vs. fed group; #first dose vs. last dose.
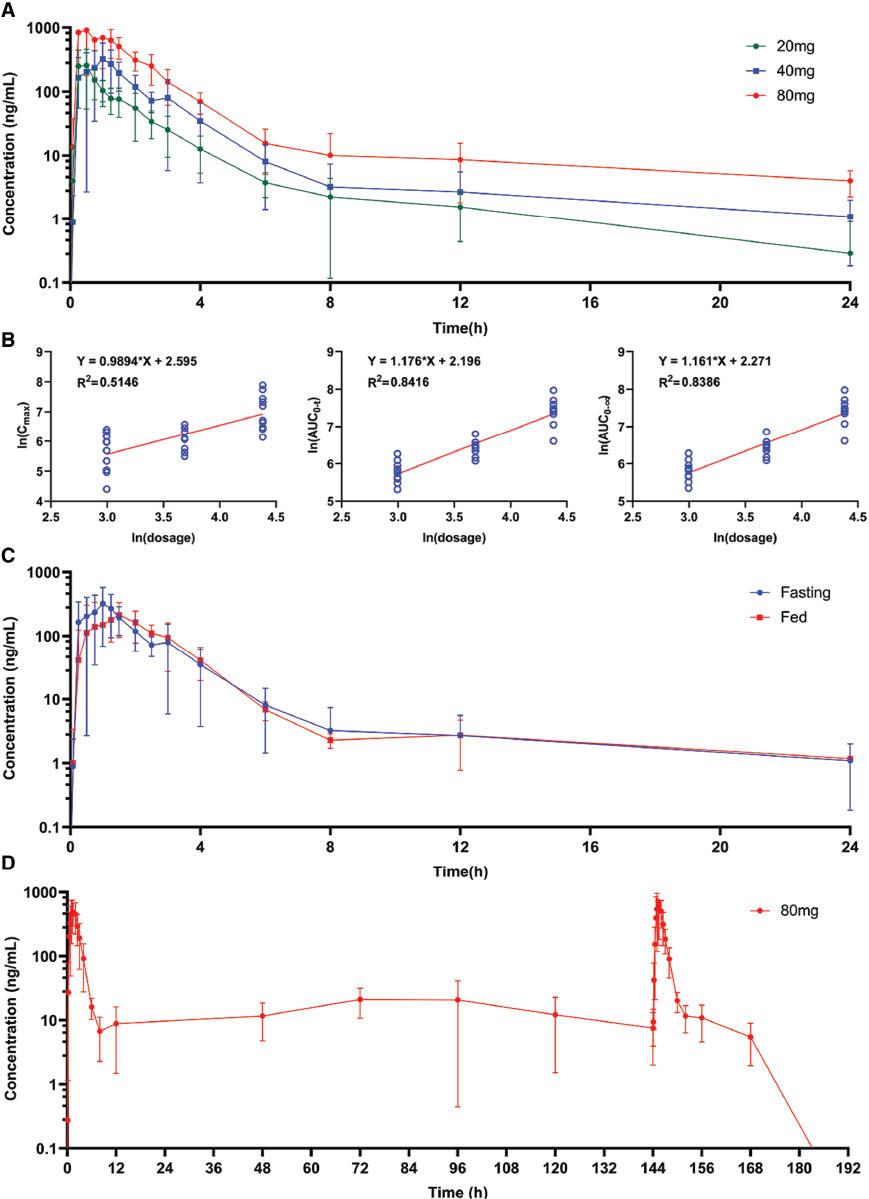
PK profile of Topiroxostat. A. Mean plasma concentration-time curve of Topiroxostat single ascending dose arm; B. The linear relation of single ascending dose and main PK parameter; C. Mean plasma concentration-time curve of Topiroxostat food effects arm; D. Mean plasma concentration-time curve of Topiroxostat multiple dose arm. (Bar: SD)
Additionally, PK parameters in the 20 mg group were associated with the characteristics of participants ( Table 3 ). Cmax was associated with age (P = 0.019). Meanwhile, AUC0-t, AUC0-∞, and CL/F were associated with height (P < 0.001) and weight (P = 0.008, 0.002, 0.002, respectively). In addition, t1/2, MRT0-t, MRT0-∞, and Kel were associated with BMI (P = 0.006, 0.048, 0.029, and 0.006, respectively). CL/F was associated with sex (P = 0.032). Vz/F was associated with sex in the 80 mg group (P = 0.032). However, no significant associations were observed in the 40 mg group.
Correlation of Characteristics of Participants, PD Parameters, and PK Parameters in the Single-dose Arm
| PK Parameter | 20 mg (N = 10), R2 | 40 mg (N = 10), R2 | 80 mg (N = 10), R2 | Total (N = 30), R2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Height | Weight | BMI | Sex | Age | Height | Weight | BMI | Sex | Age | Height | Weight | BMI | ΔECmax | ΔAUEC | |
| Cmax (ng/mL) | 0.548 | 0.019 | 0.060 | 0.166 | 0.676 | 1.000 | 0.701 | 0.711 | 0.751 | 0.260 | 0.841 | 0.803 | 0.614 | 0.580 | 0.489 | 0.040 | 0.024 |
| AUC0-t (h·ng/mL) | 0.056 | 0.328 | <0.001 | 0.008 | 0.651 | 0.421 | 0.934 | 0.530 | 0.365 | 0.881 | 0.310 | 0.960 | 0.192 | 0.293 | 0.829 | 0.001 | <0.001 |
| AUC0-∞ (h·ng/mL) | 0.032 | 0.293 | <0.001 | 0.002 | 0.489 | 0.310 | 0.907 | 0.410 | 0.293 | 0.960 | 0.421 | 0.960 | 0.250 | 0.310 | 0.803 | 0.001 | <0.001 |
| Tmax (h) | 0.762 | 0.590 | 0.915 | 0.564 | 0.089 | 0.952 | 0.605 | 0.602 | 0.173 | 0.476 | 0.675 | 0.227 | 0.601 | 0.670 | 0.328 | 0.094 | 0.064 |
| t1/2 (h) | 1.000 | 0.960 | 0.751 | 0.283 | 0.006 | 0.310 | 0.676 | 0.867 | 0.627 | 0.726 | 0.151 | 0.829 | 0.590 | 0.803 | 0.829 | 0.545 | 0.400 |
| MRT0-t (h) | 0.690 | 0.174 | 0.365 | 0.841 | 0.048 | 0.690 | 0.244 | 0.801 | 0.328 | 0.276 | 0.595 | 0.751 | 0.226 | 0.258 | 0.987 | 0.026 | 0.009 |
| MRT0-∞ (h) | 0.690 | 0.214 | 0.405 | 0.934 | 0.029 | 0.548 | 0.726 | 0.814 | 0.829 | 0.987 | 1.000 | 0.777 | 0.393 | 0.328 | 0.960 | 0.181 | 0.084 |
| CL/F (L/h) | 0.032 | 0.293 | <0.001 | 0.002 | 0.489 | 0.310 | 0.907 | 0.410 | 0.293 | 0.960 | 0.421 | 0.960 | 0.250 | 0.310 | 0.803 | 0.129 | 0.120 |
| Vz/F (L) | 0.421 | 0.467 | 0.260 | 0.776 | 0.074 | 0.421 | 0.627 | 0.907 | 0.726 | 0.803 | 0.032 | 0.489 | 0.077 | 0.328 | 0.603 | 0.730 | 0.995 |
| Kel (1/h) | 1.000 | 0.960 | 0.751 | 0.283 | 0.006 | 0.310 | 0.676 | 0.867 | 0.627 | 0.726 | 0.111 | 0.947 | 0.565 | 0.815 | 0.751 | 0.557 | 0.408 |
| AUC_%Extrap (%) | 0.310 | 0.603 | 0.054 | 0.220 | 0.726 | 0.548 | 0.701 | 0.600 | 0.556 | 0.580 | 0.310 | 0.244 | 0.614 | 0.987 | 0.701 | 0.096 | 0.110 |
PK profiles showed significant associations with PD characteristics. As shown in Figure 3A , 80 mg Topiroxostat demonstrated stronger effects than 20 or 40 mg (ANOVA, P < 0.001, F = 14.42; Table 4 ). Notably, AUC0-t and AUC0-∞ showed a significant correlation of ΔECmax (P = 0.001, 0.001) and ΔAUEC (P < 0.001, 0.001; Table 3 ). In addition, a potential linear relationship was observed between dose and ΔECmax or ΔAUEC (R2 = 0.4484 and 0.4797; Figure 3C, D ).
Blood Uric Acid Levels Among Groups
| PD Parameter | 20 mg (N = 10, mean ± SD) | 40 mg (N = 10, mean ± SD) | 80 mg (N = 10, mean ± SD) | P 3 | F4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fasting | Fed | P 1 | Fed/fasting2 | Single Dose | Multi-Dose | ||||
| ΔECmax | −30.20 ± 8.53 | −34.70 ± 11.82 | −63.10 ± 13.00 | <0.001 | 1.82 | −57.10 ± 14.81 | −154.30 ± 24.59 | <0.001 | 14.42 |
| ΔAUEC | −360.60 ± 178.60 | −541.20 ± 213.65 | −953.40 ± 218.18 | <0.001 | 1.76 | −940.80 ± 293.79 | −19104.60 ± 4178.33 | <0.001 | 16.14 |
1, 40 mg fasting group vs. 40 mg fed group; 2, BF/AF: fasting level/fed level; 3, ANOVA P value among 20 mg, 40 mg, and 80 mg fasting single dose; 4, ANOVA F value among 20 mg, 40 mg, and 80 mg fasting single dose. (ΔECmax, change in maximum of uric acid effective concentration; ΔAUEC: change in area under the uric acid concentration-time curve)
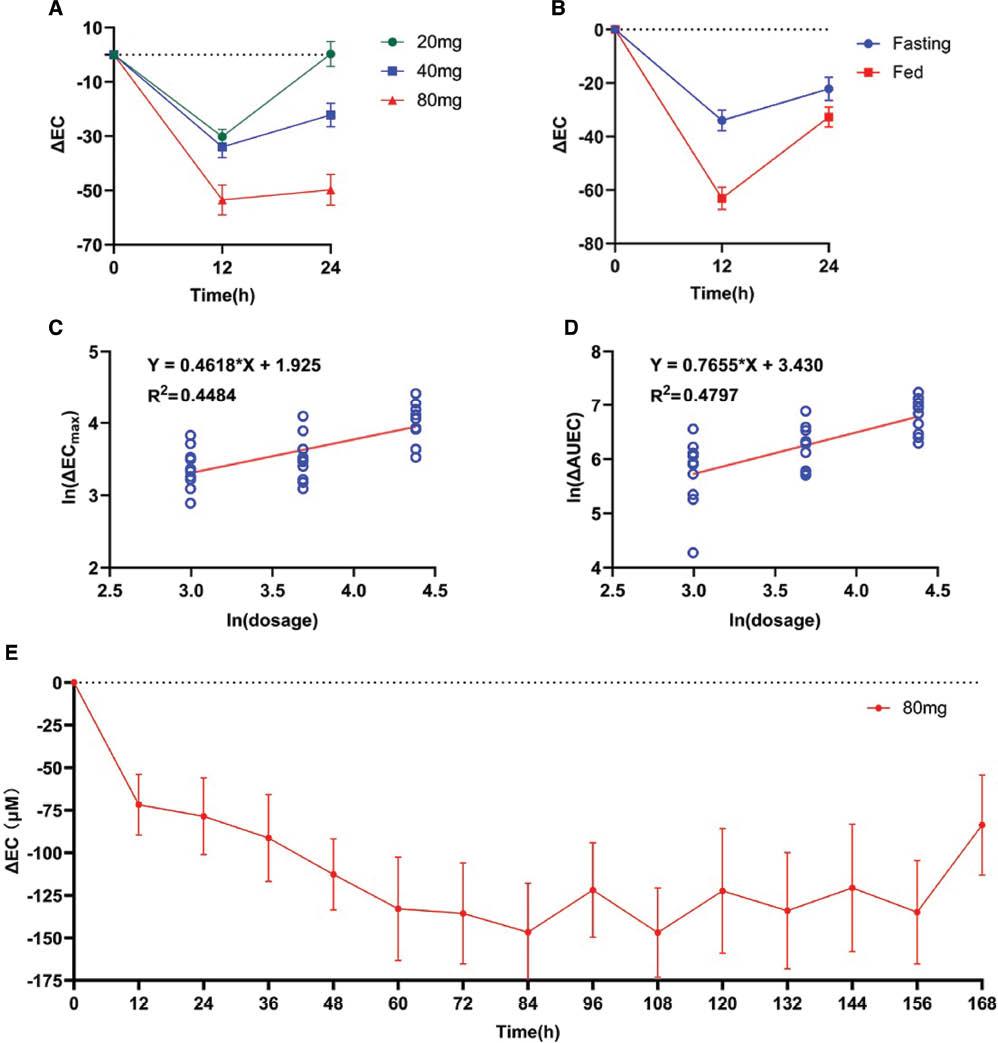
PD profile of Topiroxostat. A. ΔEC-time curve of Topiroxostat single ascending dose arm; B. ΔEC-time curve of Topiroxostat food effects arm; C, D. The linear relation of single ascending dose and main PD parameter; E. ΔEC-time curve of Topiroxostat multiple dose arm. (ΔEC, delta effective concentration; ΔAUEC: delta area under the plasma concentration–time curve). (Bar: SD)
PK and PD characteristics in the multiple-dose arm
Concentration-time curves of three doses are shown in Figure 2D . With respect to those in the first administration and last administration in the multiple-dose arm, Css_max (673.70 ± 205.74 vs. 1032.40 ± 488.21, P = 0.046), t1/2 (1.73 ± 0.85 vs. 5.82 ± 4.41, P = 0.010), MRT0-t (2.33 ± 0.49 vs. 3.49 ± 0.81, P = 0.001), and Vz/F (154.67 ± 54.46 vs. 450.81 ± 239.83, P = 0.001) increased, whereas Kel (0.46 ± 0.15 vs. 0.17 ± 0.11, P < 001) decreased ( Table 2 ). However, we did not find significant differences in Cmin in the mornings of days 3, 4, 5, and 6 (ANOVA analysis, P = 0.2649, F = 1.38).
In the PD analysis, uric acid decreased in 4 days and was maintained at a low level at days 5–7 ( Figure 3E ). Uric acid increased after the interruption of administration.
PK and PD characteristics in the food-effects arm
Concentration-time curves of the fed and fasting groups are shown in Figure 2C . As shown in Table 1 , a standardized high-fat meal decreased Cmax (478.40 ± 175.42 vs. 316.00 ± 135.81, P = 0.033) and increased Kel (0.22 ± 0.14 vs. 0.49 ± 0.22, P = 0.004).
Although the fed group showed lower Cmax levels, this group also showed stronger efficacy (ΔECmax: −63.10 ± 13.00 vs. −34.70 ± 11.82, P < 0.001, fed/fasting = 1.82; ΔAUEC: −953.40 ± 218.18 vs. −541.20 ± 213.65, P < 0.001, fed/fasting = 1.76; Table 4 ).
Safety of Topiroxostat in the three arms
To assess the safety of Topiroxostat, we recorded all AEs. As shown in Table 5 , no serious AEs occurred in this trial. Topiroxostat demonstrated good safety in the Chinese population. We analyzed the associations between AEs and PK characteristics among the three arms (Supplement Table 4) but did not find any correlations of AEs and PK profiles.
Summary of AEs
| Dosage P* | 20 mg (N = 10) | 40 mg (N = 10) | 80 mg (N = 10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | Fed | Single Dose | Multi-Dose | ||||||||
| Times | Cases (%) | Times | Cases (%) | Times | Cases (%) | Times | Cases (%) | Times | Cases (%) | ||
| Any AE | 1.000 | 13 | 7(70.00) | 4 | 3(30.00) | 1 | 1(10.00) | 25 | 7(70.00) | 21 | 8(80.00) |
| Any SAE | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) |
| Any ADR | NA | 13 | 7(70.00) | 4 | 3(30.00) | 1 | 1(10.00) | 25 | 7(70.00) | 20 | 8(80.00) |
| WBC count increased | 0.667 | 1 | 1(10.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) | 1 | 1(10.00) |
| Neutrophil count increased | 1.000 | 1 | 1(10.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) | 1 | 1(10.00) |
| Anemia | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 2 | 2(20.00) |
| Transferrin increased | 0.207 | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 2 | 2(20.00) | 0 | 0(0.00) |
| Urinary leukocyte | 0.667 | 1 | 1(10.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 2 | 2(20.00) |
| Protein urine present | 0.439 | 2 | 2(20.00) | 1 | 1(10.00) | 0 | 0(0.00) | 5 | 5(50.00) | 0 | 0(0.00) |
| Blood urine present | 0.414 | 2 | 2(20.00) | 0 | 0(0.00) | 0 | 0(0.00) | 4 | 4(40.00) | 3 | 3(30.00) |
| Urinary α1-microglobulin increased | 0.754 | 1 | 1(10.00) | 1 | 1(10.00) | 0 | 0(0.00) | 2 | 2(20.00) | 0 | 0(0.00) |
| Urinary β2-microglobulin increased | 1.000 | 1 | 1(10.00) | 1 | 1(10.00) | 0 | 0(0.00) | 1 | 1(10.00) | 0 | 0(0.00) |
| Urinary NAG increased | 0.667 | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) | 0 | 0(0.00) |
| Blood sodium decreased | 0.207 | 2 | 2(20.00) | 0 | 0(0.00) | 1 | 1(10.00) | 0 | 0(0.00) | 0 | 0(0.00) |
| Serum potassium decreased | 0.667 | 1 | 1(10.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) |
| ECG: Abnormal QRS wave complex | 0.667 | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) | 2 | 2(20.00) |
| ECG: abnormal T wave | 0.754 | 1 | 1(10.00) | 1 | 1(10.00) | 0 | 0(0.00) | 2 | 2(20.00) | 3 | 2(20.00) |
| Sinus bradycardia | 0.207 | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 2 | 2(20.00) | 0 | 0(0.00) |
| Diastolic pressure decreased | 0.667 | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 2 | 1(10.00) | 0 | 0(0.00) |
| Blood CPK increased | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) |
| Blood triglycerides increased | 0.667 | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) | 1 | 1(10.00) |
| ALT increased | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) |
| γGTP increased | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) |
| Flatulence | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) |
| Pyrexia | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) |
| Bacterial test positive | NA | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 0 | 0(0.00) | 1 | 1(10.00) |
*, P value of AE and dose; ALT, alanine aminotransferase; ADR, adverse drug reaction; AE, adverse event; CPK, creatine phosphokinase; ECG, electrocardiograph; γGTP, γ-glutamyltransferase; NA, not applicable; NAG, β-N-acetyl-D-glucosaminidase; RBC, red blood cell; SAE, serious adverse event; WBC, white blood cell.
Discussion
This is the first study reporting the safety, PD, and PK profile of Topiroxostat in Chinese participants. This phase I PK study indicated that Topiroxostat is safe and well tolerated in the Chinese population. Moreover, single daily and post cibum administration is suitable for Topiroxostat.
Single-dose Topiroxostat showed moderate variability in the Chinese population, on the basis of Cmax (36.7–59.1%) and AUC0-∞ CV (22.2–33.2%). Regarding absorption, distribution, metabolism, and excretion characteristics, Topiroxostat showed rapid absorption (Tmax < 1.6 h), rapid excretion (t1/2 = 2.49–3.72 h), and a wide distribution (Vz/F = 242.8–336.36 L). Topiroxostat is insoluble in 0.1 mol/L HCl, methanol (0.57 mg/mL), ethanol (0.48 mg/mL), and water (0.007 mg/mL), and is slightly soluble in N,N-dimethylformamide [18]. Because its fat solubility is higher than its water solubility, the high Vz/F suggested a broad organ or tissue distribution of Topiroxostat. We observed that the AUC of the first dose in the multiple-dose regimen (80 mg) was lower than the expected value ( Table 2 ). This finding might have been due to psychological changes, because the multiple-dose arm was the first trial of the entire study, and participants were likely to have felt more nervous when participating in the trial for the first time. These psychological changes might have influenced function of the intestines, the main area of absorption of Topiroxostat [19]. Cmax significantly decreased after the first dose. A comparison of our study and a PK study in healthy Japanese adults who received 60 mg Topiroxostat [20] showed no clear difference in the main PK parameters between populations. This phase I PK study provided the necessary information for Topiroxostat in the Chinese population.
In the secondary efficacy endpoint, Topiroxostat showed excellent urate-decreasing effects. A high dose (80 mg) showed stronger effects, on the basis of PD parameters (ΔEC and ΔAUEC; Figure 3A–D ). Multiple administration (80 mg, BID) steadily controlled plasma uric acid to low levels ( Figure 3E ). A previous phase III trial of Topiroxostat in hyperuricemic Japanese patients reported that Topiroxostat 120 mg/day provides non-inferior urate reduction efficacy to that of allopurinol 200 mg/day [11]. Meanwhile, the same team has also reported a dose-response relationship of Topiroxostat [21]. Our results supported that the urate-decreasing effect of Topiroxostat shows a potential dose-response relationship ( Table 4 ).
Considering the food effects, we observed that a standard high-fat diet enhanced the urate-decreasing effects of Topiroxostat ( Figure 3B , Table 4 ). Although Topiroxostat showed a dose-response relationship in the single-ascending-dose arm, we did not observe higher drug exposure in the fed group ( Table 2 , Figure 2C ). Luo et al. have predicted the PK and PD of Topiroxostat in humans according to a physiologically based PK model [19]. Their results have indicated that Topiroxostat does not penetrate and accumulate well in most organs/tissues (tissue-to-blood partition coefficient < 1.0) [19]. However, Topiroxostat shows a broad organ/tissue distribution [19]. We presumed that this distribution led to high total Topiroxostat accumulation, thus resulting in high Vz/F. Adipose tissue has been found to show the highest tissue-to-blood partition coefficient [19]. Therefore, we presumed that the standard high-fat diet enhanced the effects of Topiroxostat by increasing its the organ/tissue accumulation. The long residence time of Topiroxostat might be the main cause of its pharmacological activity [19]. More evidence should be gathered to demonstrate this hypothesis. Overall, post cibum administration increased the efficacy of Topiroxostat in the Chinese population.
In analyzing the safety of Topiroxostat, we did not use a placebo group in this trial; however, all AEs observed in this trial were mild. In addition, no potential dose-toxicity relationship was observed between PK and AE. However, renal or cardiac relative toxicity were the main AEs. Nevertheless, Horino et al. have reported that Topiroxostat shows renoprotective effects in patients with chronic kidney disease [14]. Additionally, Sugiyama et al. have reported that Topiroxostat may not cause QT-interval prolongation [20]. Furthermore, a large population phase III study of Topiroxostat has shown that a dose of 120 mg/day is well tolerated [11]. The incidence of overall AEs with 120 mg/day Topiroxostat were similar to those of allopurinol at 200 mg/day [11]. A long-term-safety observational study has shown no specific problems regarding the safety of Topiroxostat after its approval [22]. Other phase II studies have also indicated Topiroxostat’s good safety and tolerance [21–23]. Overall, Topiroxostat is considered a safe and effective drug for treating gout and hyperuricemia in daily practice. In the future, we will confirm the safety and effects of Topiroxostat under actual conditions in the Chinese population. Febuxostat, a xanthine oxidoreductase inhibitor, is widely used in clinical practice. According to one report, Febuxostat, compared with allopurinol, poses a risk of cardiovascular death in patients with gout [10]. Topiroxostat did not demonstrate cardiovascular toxicity in our trial, in agreement with findings from previous studies [20, 23, 24]. Moreover, Topiroxostat shows a lower incidence rate of gouty arthritis than febuxostat [23, 25]. Topiroxostat might be more beneficial in patients with hyperuricemia who are experiencing gouty arthritis. In contrast, Febuxostat elicits a more rapid decrease in serum uric acid than Topiroxostat in patients with cardiovascular disease and hyperuricemia [26]. Febuxostat has stronger antioxidant effects than Topiroxostat in patients with hyperuricemia and chronic kidney disease [27]. Thus, Topiroxostat and Febuxostat have differing advantages and adaptation effects.
This trial has two potential limitations. First, we were unable to explain the PK and PD relationships with food effects. However, a PK/PD model such as the physiologically based PK model may be considered to predict their relationship. Second, this study was based on a healthy Chinese population. Considering the differences in PK profiles between the Japanese and Chinese populations, further PK/PD trials in patients with hyperuricemia, older people, and other populations should be performed in the future.
Conclusion
The PK profile of Topiroxostat in the Chinese population was assessed in this study. No severe AEs were observed in any participants. Topiroxostat had good safety and tolerance in the Chinese population. Additionally, this phase I PK and PD study of Topiroxostat supports that post cibum administration is suitable for Topiroxostat in the Chinese population.


