1 INTRODUCTION
Polyoxometalates (POM) are oxo clusters of transition metal ions, such as Mo, W, V, Nb, and Ta, forming a variety of structures. POMs have different sizes and shapes which may allow for the inclusion of other anions. In the structure of POMs, one or more of the metal oxoanions can be substituted[1–3].
Polyoxometalates (POM) have potential applications in various fields of science and technology, catalytic, magnetic, and electrochemical, in light of their properties such as thermal stability, redox activity, solubility in polar and non-polar solvents[4,5]. POMs have dominated medicinal chemistry for their intriguing antiviral and anti-tumor activities. Among the POMs, polyoxovanadate compounds (POVs) are known for their extensive applications in several fields such as chemical, physical and biological sciences. Vanadium plays an important role in biological systems and biochemistry[6,7]. Although POVs generally contain vanadium in the +5 oxidation state, recent reports have described POVs with two oxidation states, V(IV) and V(V). Several decavanadate compounds are stable under physiological conditions. The stability of POV under the given experimental conditions requires due consideration in the research on the effects of POV[1,8].
Decavanadate compounds are formed in the pH range 6-8 and show a potential interest in several fields such as catalysis, nanotechnology, electrochemistry, materials science, anticancer, antibacterial and antiviral activity[1,8–10]. In the present work, we will focus on the synthesis, structure and Hirshfeld surface analysis of a new decavanadate compound: Na2[H4V10O28]·14H2O.
2 EXPERIMENTAL
2.1 Materials and Measurements
All solvents and reagents were obtained from commercial sources and used without further purification.
2.2 Synthesis of Na2[H4V10O28]·14H2O Compound
The compound Na2[H4V10O28]·14H2O was obtained from a mixture of 0.6 g of V2O5 (99.99%, FLUKA) and 0.5 g of NaVO3 (Prolabo, 98%) in 100 mL of pure water. The mixture obtained was placed under magnetic stirring and heating for approximately 2 hours. After five days of slow evaporation at room temperature, orange good quality crystals were obtained.
2.3 Hirshfeld Surface Study
Hirshfeld surfaces (HS) were constructed to represent the asymmetric unit of the compound and the analysis was performed using the Crystal-Explorer program[11].
2.4 X-ray Crystallography
An Enraf-Nonius CAD4[12] 4-circle diffractometer was used to collect the diffracted intensities (λ = 0.71067 Å). The resolution of the structure was performed by the direct method using the SHELXS-97[13] program and the refinement was performed by the least-squares method using SHELXL-2014[14]. Hydrogen atoms were attached using the HFIX instruction. The absorption correction was performed by psi-scan[15]. All the figures of the structure have been represented by the DIAMOND software[16].
Crystal data, data collection, and structural refinement details are summarized in Table 1.
Crystallographic Characteristics, X-ray Data Collection, and Structure-Refinement Parameters of Na2[H4V10O28]·14H2O Compound
| Crystal Data | |
| Chemical formula | Na2[H4V10O28]·14H2O |
| Formula weight (g;mol−1) | 1263.67 |
| Crystal system, space group | Triclinic, P-1 |
| T (K) | 298(2) |
| a b c (Å) | 11.282(5), 10.424(3), 8.502(1) |
| α, β, γ (°) | 112.81(2), 87.25(2), 111.49(5) |
| V(Å3) | 852.4(5) |
| Z | 2 |
| Radiation λ (Å) | MoKα 0.71073 |
| Crystal size (mm3) | 0.68×0.54×0.39 |
| μ (mm−1) | 2.771 |
| F(000) | 682 |
| Data Collection | |
| Diffractometer | Enraf-Nonius CAD4 |
| Absorption correction | Ψ-scan |
| Tmin,Tmax | 0.181,0.339 |
| Range for data collection (°) | 2.3≤θ≤ 27 |
| h, k, l ranges | -14≤ h ≤14, -12≤ k ≤13, -10≤ l ≤1 |
| Scan mode | ω/2θ |
| No. of measured, independent, and observed | 4205, 3713, 3304 |
| [I >2σ(I)] reflections | |
| Rint | 0.016 |
| Refinement | |
| R1 [F2 > 2 σ (F2)] | 0.033 |
| wR2(F2) | 0.1 |
| S | 1.09 |
| No. of parameters | 301 |
| Maximum residual electron density Δρmax (e.Å−3) | 0.642 |
| Minimum residual electron density Δρmin (e.Å−3) | -0.705 |
3 RESULTS AND DISCUSSION
3.1 Crystal Structure
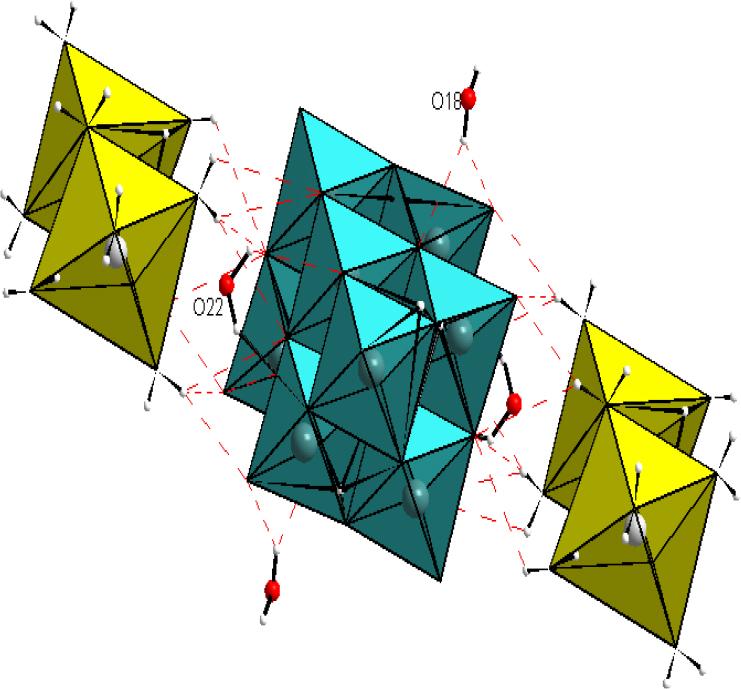
The formula unit of Na2[H4V10O28]·14H2O compound was formed by an acidic decavanadate group [H4V10O28]2-, a Na2(H2O)10 2+ dimer, and four molecules of water. The structure of the decavanadate group [V10O28]6- is formed by ten VO6 octahedra interconnected by sharing of edges[17,18](Figure 1).
The projection, according to c, of the structure of the compound Na2[H4V10O28]·14H2O shows that the decavanadate groups [H4V10O28]2-, the Na2(H2O)10 2+ dimers, and the water molecules stack in layers parallel to the (010) plane (Figure 2).

(A) Projection of the structure of Na2[H4V10O28]·14H2O compound according to the caxis, (B) Decavanadate group, (C) Na2(H2O)10 2+dimer.
The cohesion of the structure is ensured by O-H…O hydrogen bonds and van der Waals interactions (Figure 3). These bonds are weak according to Brown’s criterion[19] (Table 2). The comparison of the studied structure with the two structures (NH4)4Li2[V10O28]·10H2O and Na5.22Li0.78[V10O28]·20H2O: studied by KSIKSI et al.[18,20], shows that sodium and lithium form dimers in the structure studied and the compound (NH4)4Li2[V10O28]·10H2O. The cohesion in these two structures is ensured only by hydrogen bonds and van der Waals interactions. In the structure of Na5.22Li0.78[V10O28]·20H2O, sodium forms infinite chains. The cohesion of the decavanadate structure Na5.22Li0.78[V10O28]·20H2O is ensured by the pooling of oxygen vertices, ridges and vertices, and by van der Waals interactions. Sodium forms chains, interconnected by the pooling of vertices, edges and faces. These strong bonds can provide better stability to decavanadates compounds, which encourages the synthesis of decavanadates compounds containing sodium.
Hydrogen Bonds of Na2[H4V10O28]·14H2O Compound
| D—H…A | d(D—H) | d(H…A) | d(D…A) | <D—H…A> |
|---|---|---|---|---|
| O20-H5…O13 | 0.901 | 1.902 | 2.800 | 173.97 |
| O15-H15A…O1 | 0.776 | 2.114 | 2.719 | 135.00 |
| O15-H15A…O6i | 0.776 | 2.168 | 2.665 | 122.37 |
| O15-H15A…O8i | 0.776 | 2.268 | 2.682 | 114.28 |
| O15-H15A…O11 | 0.776 | 2.299 | 2.668 | 110.16 |
| O16-H16A…O8ii | 0.873 | 2.203 | 3.040 | 160.52 |
| O16-H16B…O15iii | 0.811 | 2.048 | 2.853 | 172.50 |
| O17-H17A…O2 | 0.813 | 2.058 | 2.866 | 172.12 |
| O17-H17B…O11 | 0.848 | 1.987 | 2.829 | 172.09 |
| O18-H18A…O1iii | 0.769 | 2.252 | 2.954 | 152.11 |
| O18-H18A…O10iv | 0.769 | 2.539 | 3.110 | 132.27 |
| O18-H18B…O9 | 0.869 | 2.039 | 2.869 | 159.32 |
| O19-H19A…O3 | 0.888 | 1.914 | 2.799 | 174.07 |
| O19-H19B…O8iv | 0.871 | 2.306 | 2.979 | 134.05 |
| O19-H19B…O10iv | 0.871 | 2.310 | 3.054 | 143.32 |
| O20-H20A…O12v | 0.860 | 2.248 | 2.982 | 143.22 |
| O21-H21A…O14vi | 1.063 | 2.187 | 3.222 | 164.03 |
| O21-H21B…O11 | 0.909 | 2.490 | 3.079 | 122.83 |
| O21-H21B…O13I | 0.909 | 2.336 | 3.093 | 140.60 |
| O21-H21B…O15i | 0.909 | 2.341 | 3.161 | 150.04 |
| O22-H22A…O2 | 0.855 | 2.485 | 3.031 | 122.49 |
| O22-H22A…O7vii | 0.855 | 2.126 | 2.863 | 144.07 |
| O22-H22B…O3 | 0.884 | 2.440 | 3.038 | 125.43 |
| O22-H22B…O10iv | 0.884 | 2.255 | 2.924 | 132.32 |
Symmetry codes : i: -x+1, -y+1, -z+1, ii: x+1, y+1, z+1, iii: x, y+1, z+1, iv: -x+1, -y+2, -z+1, v: -x+2, -y+2, -z+2, vi: x-1, y-1, z, vii: -x+1, -y+1, -z.
3.2 Hirshfeld Surface Analysis of Na2[H4V10O28]·14H2O
The Hirshfeld surface of the decavanadate compound studied in normal mode dnorm is shown in Figure 4A. This figure shows that the main interactions are between the surfaces H…H and O…H/H…O[21,22]. The structure of the compound Na2[H4V10O28]·14H2O is dominated by the interactions O…H/H…O (59.5 ℅), H…H (14.9 ℅), and V…O/O…V contacts (11.8 ℅) (Figures 4 A, B and C). The O…O contacts represent 9.3 ℅.
4 CONCLUSION
A new compound decavanadate, Na2[H4V10O28]·14H2O, was synthesized by slow evaporation at room temperature. The structure is formed by the decavanadate groups, Na2(H2O)10 2+ dimers, and water molecules. The cohesion of the structure is ensured by hydrogen bonds and van der Waals interactions. The study of the HS surface shows that the structure is dominated by O…H/H…O, H…H and V…O/O…V contacts.